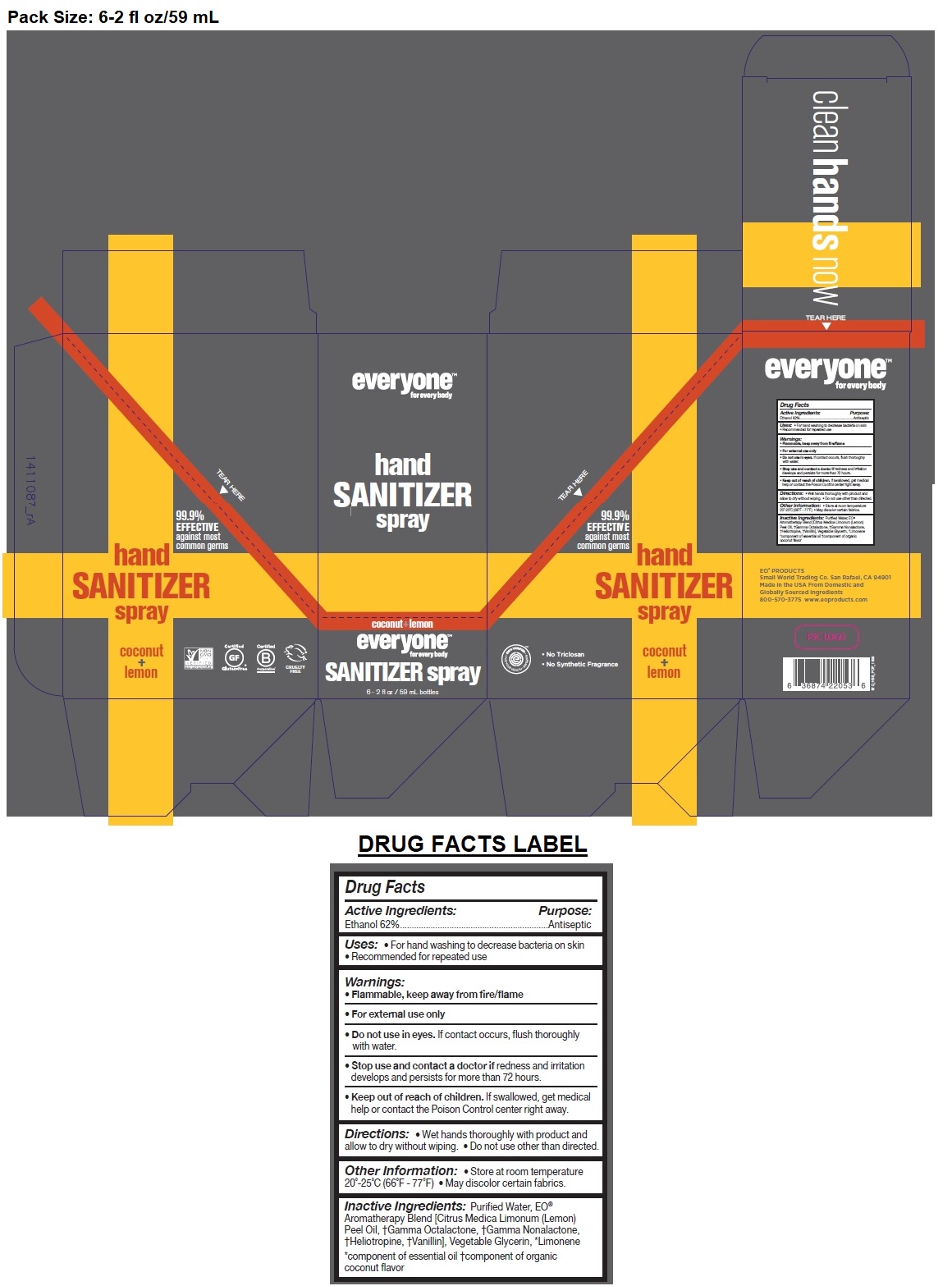
Label: EVERYONE HAND SANITIZER COCONUT LEMON- alcohol spray
-
NDC Code(s):
54748-301-02,
54748-301-05,
54748-301-07,
54748-301-08, view more54748-301-09, 54748-301-12
- Packager: EO Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients:
- Purpose:
- Uses:
- Warnings:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
- Inactive Ingredients:
- Other Information:
-
SPL UNCLASSIFIED SECTION
99.9% EFFECTIVE against most common germs
- No Disodium EDTA
clean hands now
no parabens / no synthetic fragrances / no triclosan
made with moisturizing ingredients
Better for you means better for everyone. That’s why Everyone doesn’t believe in using fake ingredients that are impossible to pronounce. Inside every bottle are plant-based moisturizers. Soothing plant extracts, pure essential oils, and goodness you can trust. Some things are better when shared, including your cleansing routine.
NOPE!
no synthetic fragrance
no triclosan
no phthalates
no animal testing
YEP!
moisturizing plant extracts
pure essential oils
sugar cane derived alcohol
zero-waste manufacturing
DIRECTIONS: Never run on empty. Just use our Refill Size to fill your previously-used 2oz / 8oz sanitizer bottles. Less plastic means less waste, better savings, and a more fulfilling clean.
EO® PRODUCTS
Small World Trading Co. San Rafael, CA 94901
Made in the USA From Domestic and Globally Sourced Ingredients
800-570-3775 www.eoproducts.com
- Packaging
-
INGREDIENTS AND APPEARANCE
EVERYONE HAND SANITIZER COCONUT LEMON
alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54748-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEMON OIL (UNII: I9GRO824LL) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) PIPERONAL (UNII: KE109YAK00) VANILLIN (UNII: CHI530446X) GLYCERIN (UNII: PDC6A3C0OX) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54748-301-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/07/2013 02/15/2017 2 NDC:54748-301-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2018 3 NDC:54748-301-09 6 in 1 PACKAGE 08/31/2018 3 NDC:54748-301-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:54748-301-07 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/12/2020 5 NDC:54748-301-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/12/2020 6 NDC:54748-301-12 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/07/2013 Labeler - EO Products, LLC (786611210) Establishment Name Address ID/FEI Business Operations EO Products, LLC 786611210 manufacture(54748-301)