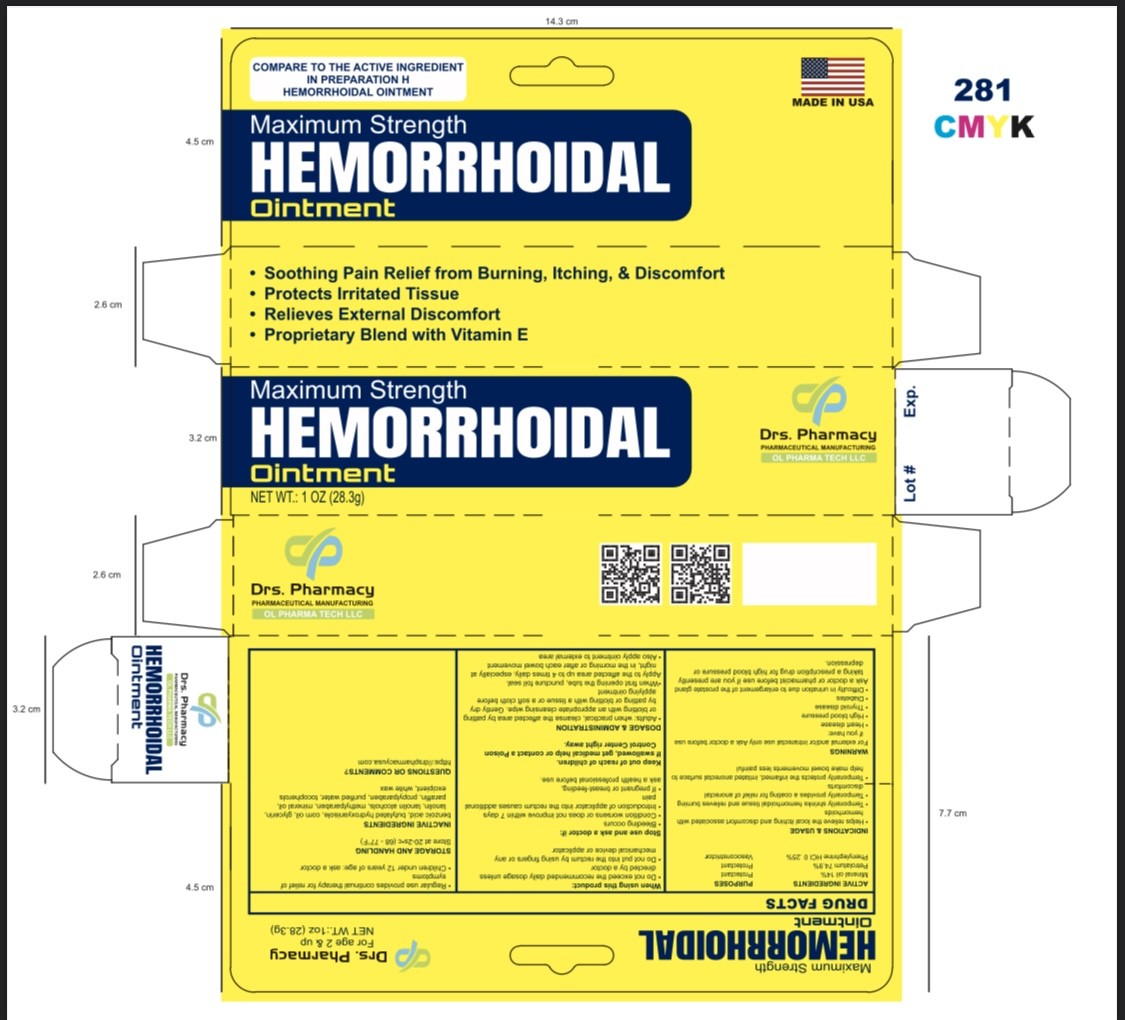
Label: DRS PHARMACY HEMORRHOIDAL- phenylephrine hcl ointment
- NDC Code(s): 80489-956-01
- Packager: OL PHARMA TECH, LLC Drs PHARMACY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
-
USES
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporarily shrinks hemorrhoidal tissue and relieves burning
- temporarily provides a coating for relief of anorectal discomforts
- aids in protecting irritated anorectal areas
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
- WARNINGS
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- Ask a doctor or pharmacist before use if you are
- WHEN USING THIS PRODUCT
- STOP USING AND ASK YOUR DOCTOR IF
- IF PREGNENT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying gel.
- when first opening the tube, puncture foil seal with top end of cap
- apply externally to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
- children under 12 years of age: ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRS PHARMACY HEMORRHOIDAL
phenylephrine hcl ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80489-956 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 749 mg in 1 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 1 g MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 140 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) PARAFFIN (UNII: I9O0E3H2ZE) CORN OIL (UNII: 8470G57WFM) GLYCERIN (UNII: PDC6A3C0OX) THYME (UNII: CW657OBU4N) WHITE WAX (UNII: 7G1J5DA97F) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) WATER (UNII: 059QF0KO0R) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80489-956-01 1 in 1 CARTON 11/01/2021 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 11/01/2021 Labeler - OL PHARMA TECH, LLC Drs PHARMACY (021170377) Registrant - OL PHARMA TECH, LLC Drs PHARMACY (021170377) Establishment Name Address ID/FEI Business Operations OL PHARMA TECH, LLC Drs PHARMACY 021170377 manufacture(80489-956)