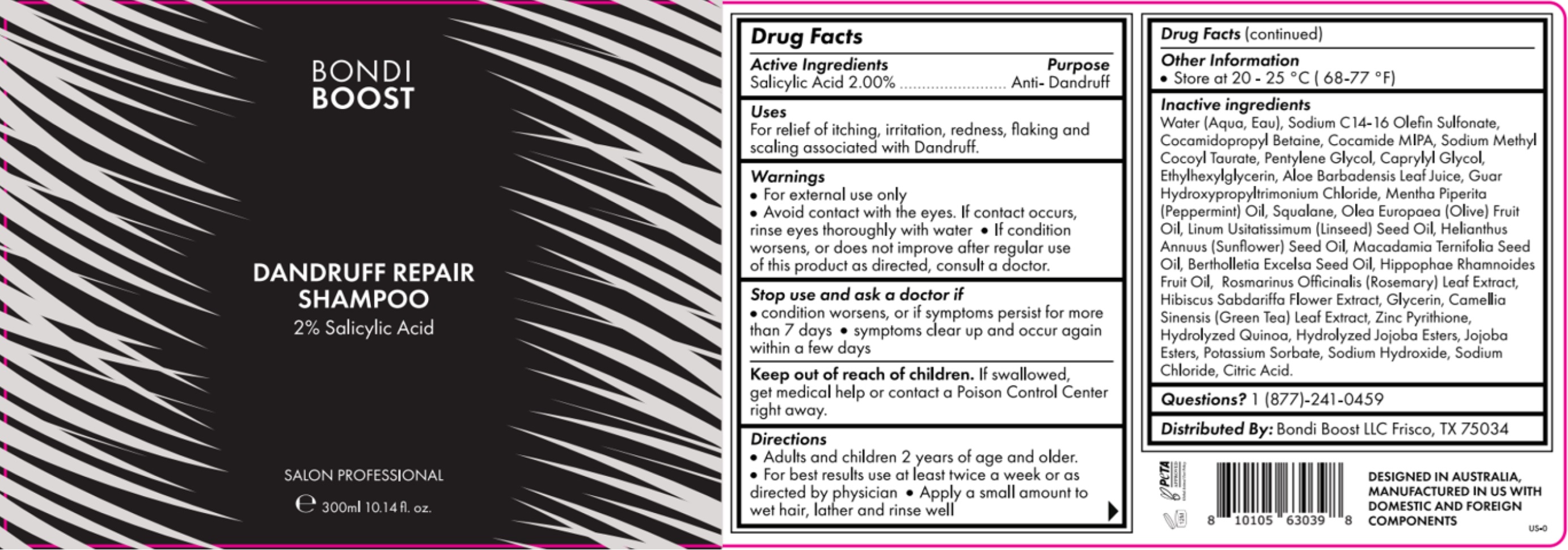
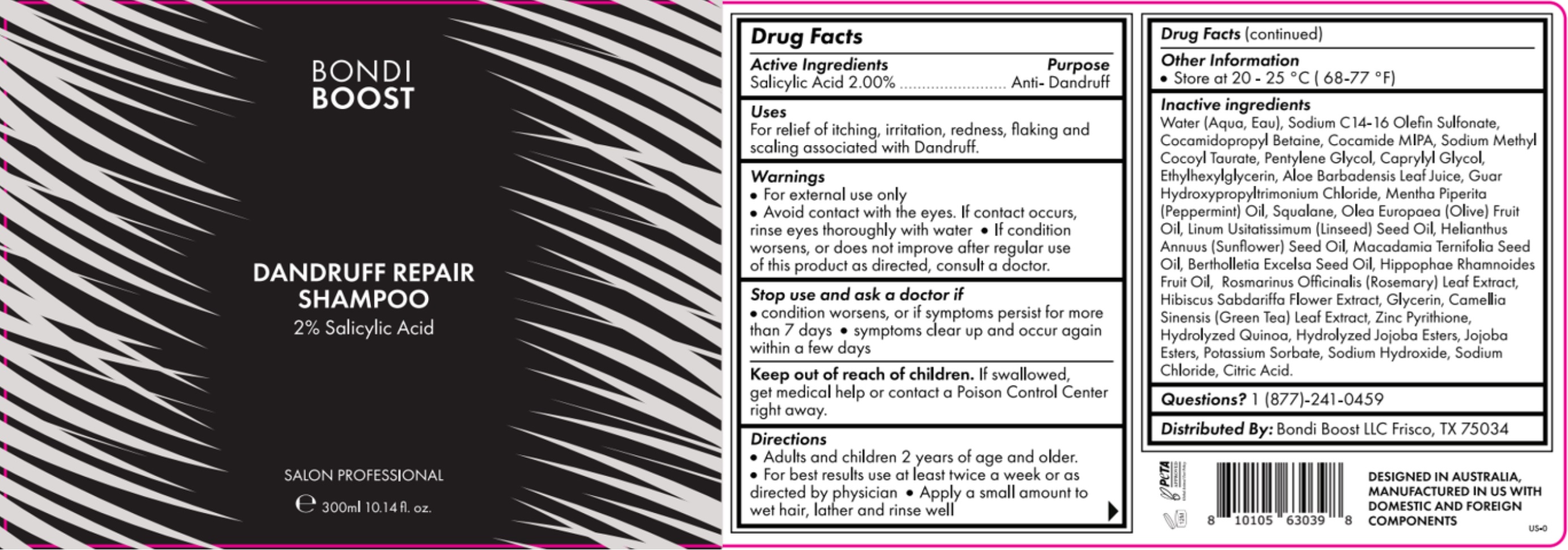
Label: S- salicylic acid shampoo shampoo
- NDC Code(s): 84169-001-01
- Packager: Bondi Boost U.s LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Stop use and ask a doctor if
- ASK DOCTOR/PHARMACIST
- DO NOT USE
- Keep Out of reach of children
- Questions?
- Warnings
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
Water (Aqua, Eau), Sodium C 14 -16 Olefin Sulfonate, Cocamidopropyl Betaine, Cocamide MIPA, Sodium Methyl Cocoyl Taurate, Pentylene Glycol, Caprylyl Glycol, Ethylhexylglycerin, Aloe Barbadensis Leaf Juice, Gaur Hydroxypropyltrimonium chloride, Mentha Piperita (Peppermint) Oil, Squalene, Olea Europaea (olive) fruit Oil, Linum Usitatissimum (Linseed) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Macadamia Ternifolia Seed Oil, Bertholletia Excelsa Seed oil, Hippophae Rhamnoides Fruit Oil, Rosmarinus Officinalis (Rosemar) LEad Extract, Hibiscus Sabdariffa Flower Extract, Glycerin, Camellia Sinensis ( Green Tea) Leaf Extract, Zinc Pyrithione, Hydrolyzed Quinoa, Hydrolyzed Jojoba Esters, Jojoba Esters, Potassium Sorbate, Sodium Hydroxide, Sodium Chloride, Citric acid.
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
S
salicylic acid shampoo shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84169-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength ROSEMARY (UNII: IJ67X351P9) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM CHLORIDE (UNII: 451W47IQ8X) OLIVE OIL (UNII: 6UYK2W1W1E) PYRITHIONE ZINC (UNII: R953O2RHZ5) BRAZIL NUT OIL (UNII: 0G89T29HO6) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (UNII: B16G315W7A) MENTHA PIPERITA (UNII: 79M2M2UDA9) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SQUALENE (UNII: 7QWM220FJH) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) LINSEED OIL (UNII: 84XB4DV00W) GLYCERIN (UNII: PDC6A3C0OX) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) PENTYLENE GLYCOL (UNII: 50C1307PZG) MACADAMIA OIL (UNII: 515610SU8C) SODIUM HYDROXIDE (UNII: 55X04QC32I) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84169-001-01 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/15/2024 Labeler - Bondi Boost U.s LLC (119222402) Establishment Name Address ID/FEI Business Operations Dhaliwal Pharmaceuticals Laboratories, LLC 116933772 manufacture(84169-001)