Label: CLEANSOL ANTIBACTERIAL WET WIPES- benzalkonium choloride cloth
- NDC Code(s): 84247-002-01, 84247-002-02
- Packager: Oceanline LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
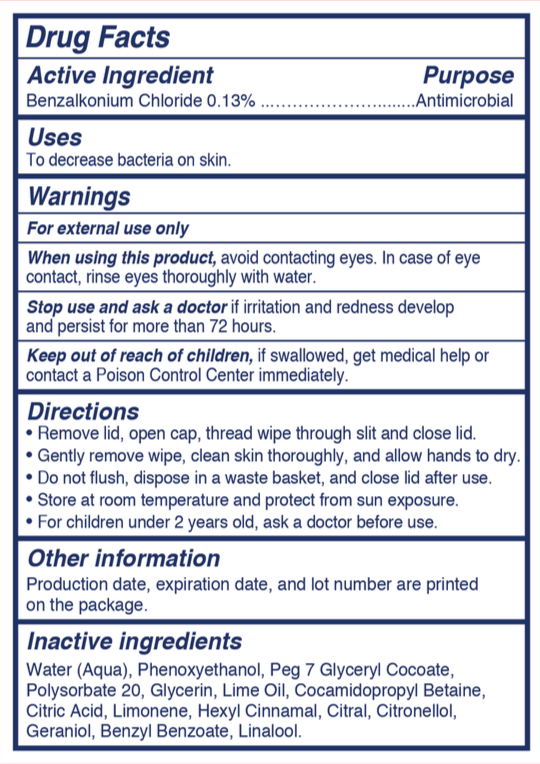
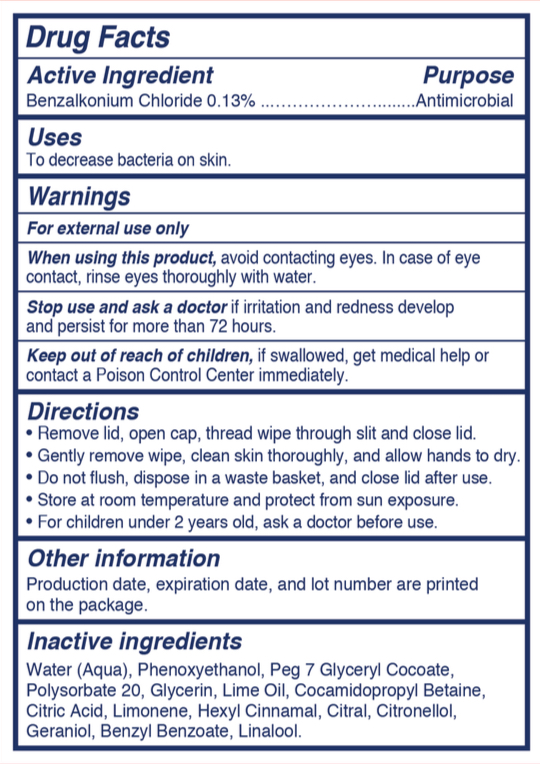
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children
-
Directions
- Remove lid, open cap, thread wipe through slit and close lid
- Gently remove wipe, clean skin thoroughly, and allow hands to dry
- Do not flush, dispose in a waste basket, and close lid after use
- Store at room temperature and protect from sun exposure
- For children under 2 years old, ask a doctor before use
- Other Information
- Inactive Ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CLEANSOL ANTIBACTERIAL WET WIPES
benzalkonium choloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84247-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) WATER (UNII: 059QF0KO0R) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIME OIL (UNII: UZH29XGA8G) GERANIOL (UNII: L837108USY) BENZYL BENZOATE (UNII: N863NB338G) CITRAL (UNII: T7EU0O9VPP) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84247-002-01 100 in 1 POUCH 05/01/2024 1 3.24 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:84247-002-02 100 in 1 CANISTER 05/01/2024 2 3.24 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2024 Labeler - Oceanline LLC (080450983)