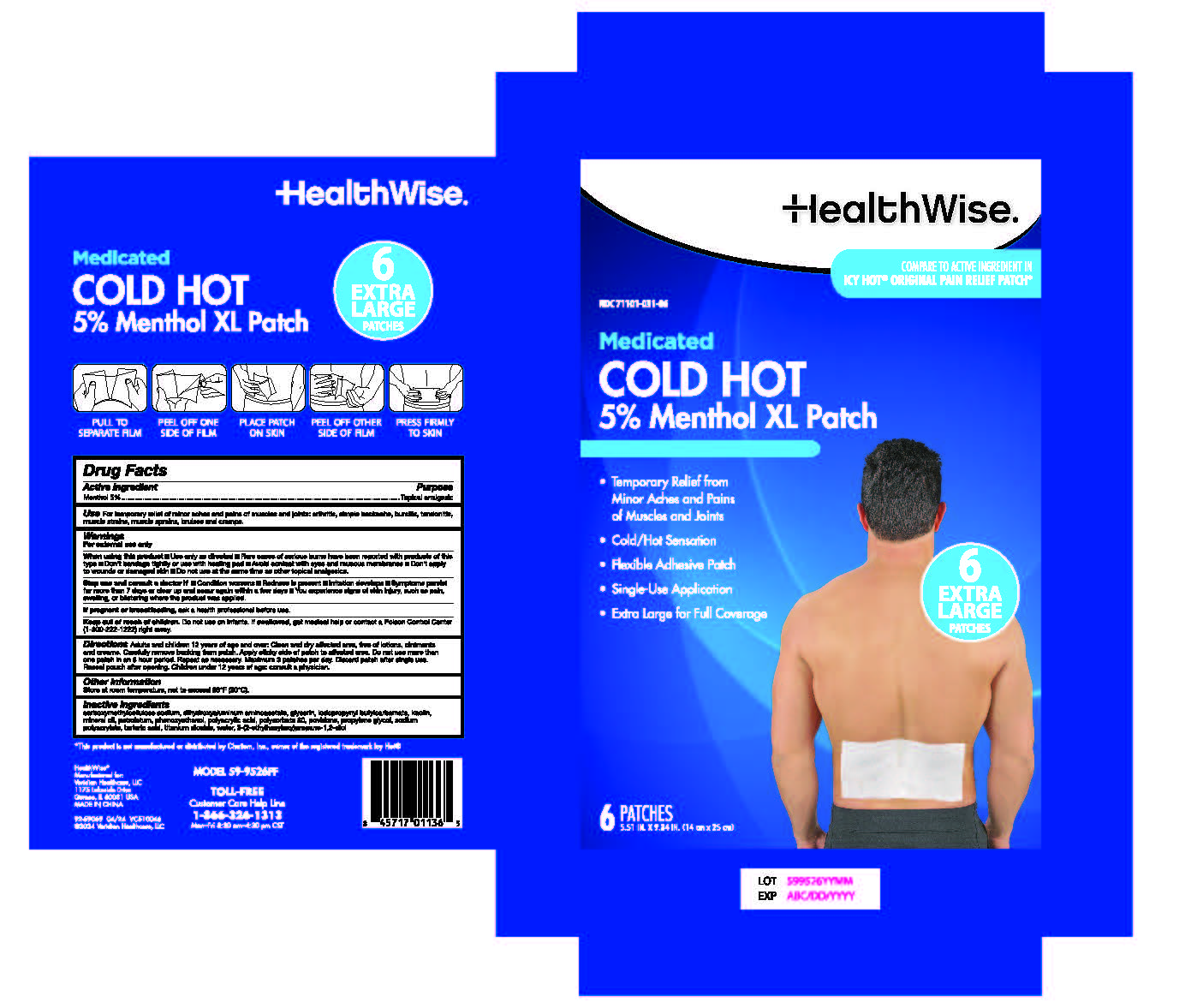
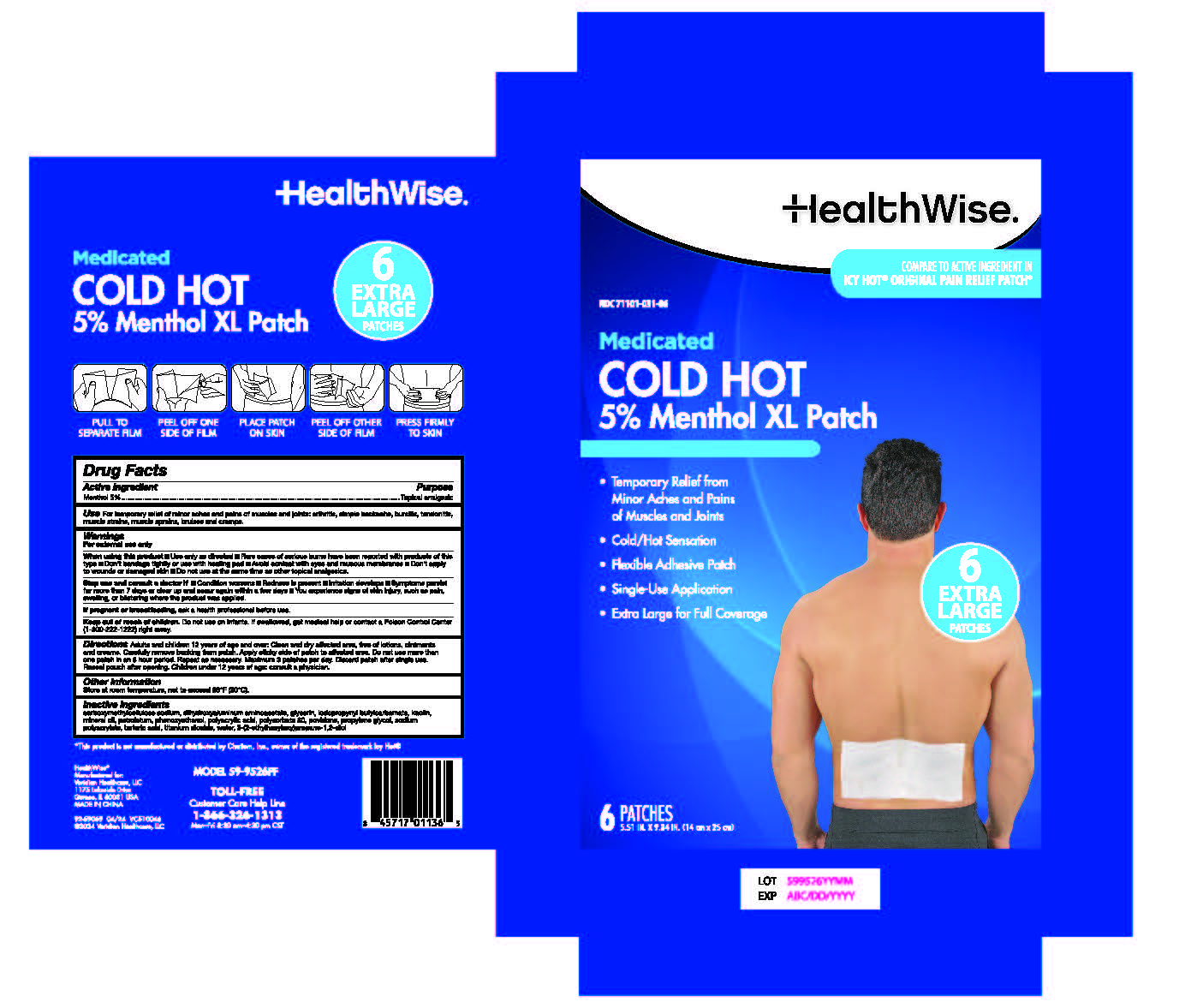
Label: HEALTHWISE MEDICATED COLD HOT 5% PATCH- menthol patch
- NDC Code(s): 71101-031-06, 71101-031-25
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
carboxymethylcellulose sodium, Dihydroxyaluminum Aminoacetate, Glycerin, iodopropynyl butylcarbamate, Kaolin, Mineral Oil, Petrolatum, Phenoxyethanol, Polyacrylic Acid, Polysorbate 80, povidone, Propylene Glycol, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Water, 3-(2-ethylhexyloxy)propane-1,2-diol
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Adults and children 12 years of age and over: Clean and dry affected area, free of lotions, ointments and creams. Carefully remove backing from patch. Apply sticky side of patch to affected area. Do not use more than one patch in an 8 hour period. Repeat as necessary. Maximum 3 patches per day. Discard patch after single use. Reseal pouch after opening.
Children under 12 years of age: consult a physician.
- PURPOSE
-
When using this product
When using this product
■ Use only as directed
■ Rare cases of serious burns have been reported with products of this type
■Don't bandage tightly or use with heating pad
■ Avoid contact with eyes and mucous membranes
■ Don't apply to wounds or damaged skin
■ Do not use at the same time as other topical analgesics.
- Stop use and ask a doctor
- If pregnant or breastfeeding
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEALTHWISE MEDICATED COLD HOT 5% PATCH
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) KAOLIN (UNII: 24H4NWX5CO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) TARTARIC ACID (UNII: W4888I119H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-031-06 6 in 1 BOX 04/01/2024 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:71101-031-25 25 in 1 BOX 04/01/2024 2 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/01/2024 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd., 529128763 manufacture(71101-031)