Label: HOSPICARE 40R ADULT BODY WIPES- wet wipes cloth
- NDC Code(s): 84215-004-01
- Packager: Freshening Paper Products(Hui Zhou)Co.Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
-
Use
●Extra-large towel size for ease of cleaning
●Strong and tear resistant for surface cleaning yet gentle on skin
●Improves patient care and satisfaction
●Eliminates odour associated with incontinence changes
●Eliminates use of harsh soaps, rough washcloths and towel drying
●Helps you stay cool, clean, comfortable and refreshed
anytime, anywhere - Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
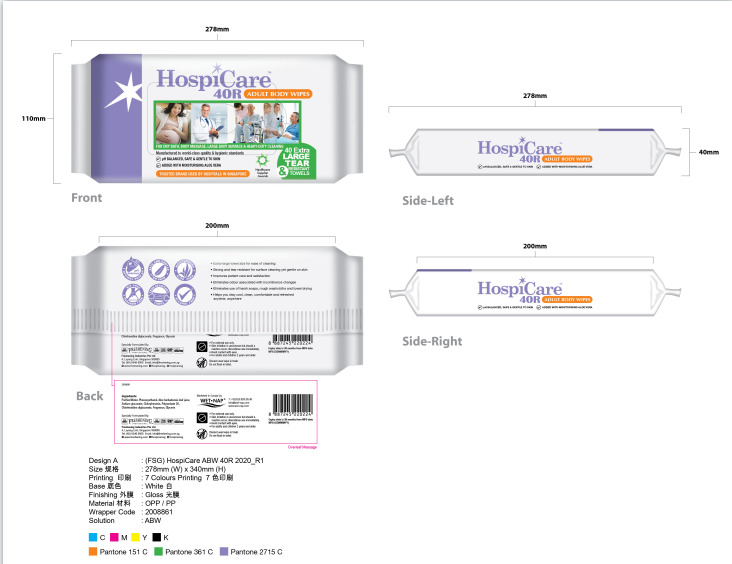
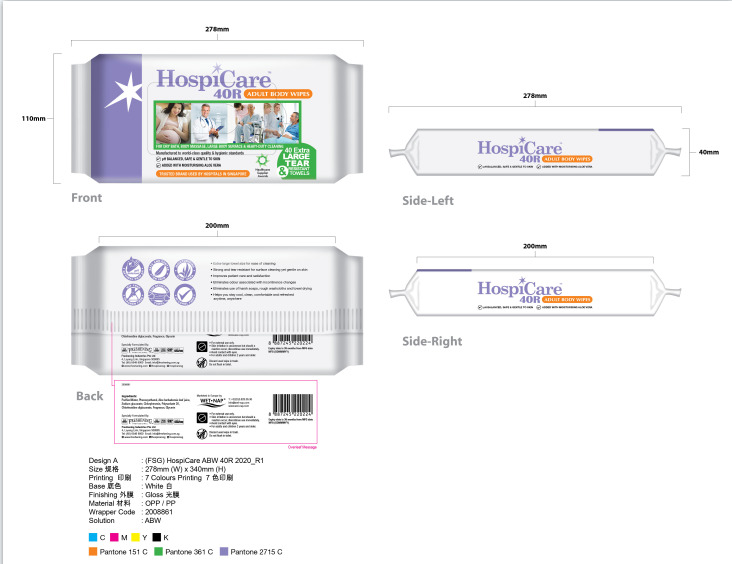
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HOSPICARE 40R ADULT BODY WIPES
wet wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84215-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 0.1 g in 100 Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM GLUCONATE (UNII: R6Q3791S76) WATER (UNII: 059QF0KO0R) CHLORPHENESIN (UNII: I670DAL4SZ) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84215-004-01 40 in 1 BAG; Type 0: Not a Combination Product 04/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/09/2024 Labeler - Freshening Paper Products(Hui Zhou)Co.Ltd (723246894) Establishment Name Address ID/FEI Business Operations Freshening Paper Products(Hui Zhou)Co.Ltd 723246894 manufacture(84215-004)