Label: NADOLOL tablet
- NDC Code(s): 68001-630-00, 68001-631-00, 68001-632-00
- Packager: BluePoint Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 9, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONPRODUCT OVERVIEW: Nadalol Tablets USP, 20mg, 40mg and 80mg - (Nadolol USP) Rx Only
-
DESCRIPTIONNadolol tablets, USP is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-( tert-butyl-amino)-3-[(5,6,7,8-tetrahydro ...
-
CLINICAL PHARMACOLOGYNadolol tablets, USP is a nonselective beta-adrenergic receptor blocking agent. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and ...
-
INDICATIONS AND USAGEAngina Pectoris - Nadolol tablets, USP is indicated for the long-term management of patients with angina pectoris. Hypertension - Nadolol tablets, USP is indicated for the treatment of ...
-
CONTRAINDICATIONSNadolol is contraindicated in bronchial asthma, sinus bradycardia and greater than first degree conduction block, cardiogenic shock, and overt cardiac failure (see - WARNINGS).
-
WARNINGSCardiac Failure - Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate ...
-
PRECAUTIONSImpaired Renal Function - Nadolol should be used with caution in patients with impaired renal function (see - DOSAGE AND ADMINISTRATION). INFORMATION FOR PATIENTS - Interruption or ...
-
ADVERSE REACTIONSMost adverse effects have been mild and transient and have rarely required withdrawal of therapy. Cardiovascular - Bradycardia with heart rates of less than 60 beats per minute occurs commonly ...
-
OVERDOSAGENadolol can be removed from the general circulation by hemodialysis. In addition to gastric lavage, the following measures should be employed, as appropriate. In determining the duration of ...
-
DOSAGE AND ADMINISTRATIONDOSAGE MUST BE INDIVIDUALIZED. NADOLOL TABLETS, USP MAY BE ADMINISTERED WITHOUT REGARD TO MEALS. Angina Pectoris - The usual initial dose is 40 mg Nadolol Tablets once daily. Dosage may be ...
-
HOW SUPPLIEDAll tablets are blue color with mottling, round, biconvex, having a score line on one side and debossed with the product code on the other. Nadolol Tablets are available in the following ...
-
SPL UNCLASSIFIED SECTIONManufactured by - BEXIMCO PHARMACEUTICALS LTD. 126, Kathaldia, Tongi, Gazipur, 1711, Bangladesh - For BluePoint Laboratories - Revised: 12/2024
-
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle LabelNDC 68001-630-00 - Nadolol Tablets, USP - 20 mg - 100 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle LabelNDC 68001-631-00 - Nadolol Tablets, USP - 40 mg - 100 Tablets - Rx only
-
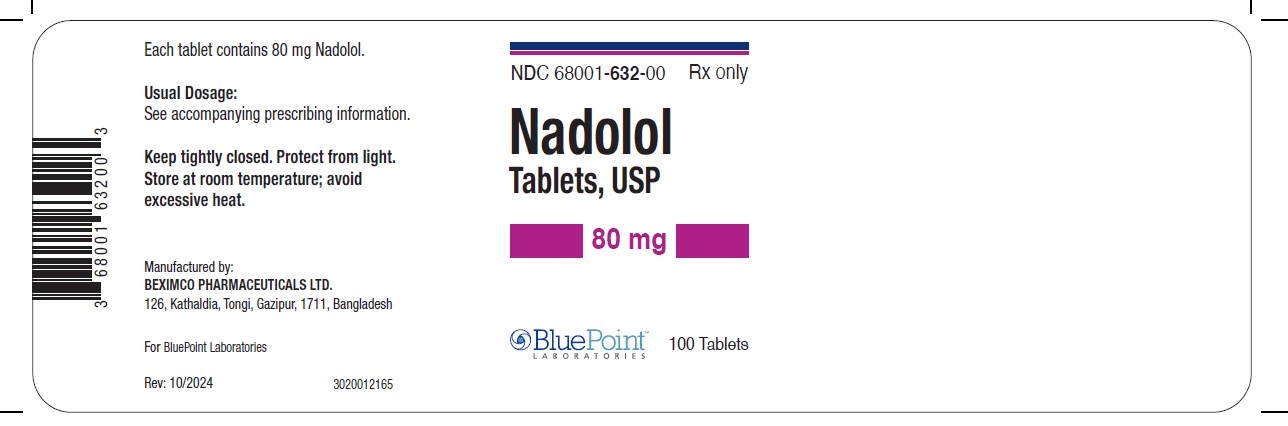
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle LabelNDC 68001-632-00 - Nadolol Tablets, USP - 80 mg - 100 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information