Label: EPHEDRINE SULFATE injection
- NDC Code(s): 65145-158-01, 65145-158-25
- Packager: Caplin Steriles Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPHEDRINE SULFATE INJECTION safely and effectively. See full prescribing information for EPHEDRINE SULFATE INJECTION. EPHEDRINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEphedrine Sulfate Injection is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosage and Administration Instructions - Ephedrine Sulfate Injection, 50 mg/mL must be diluted before administration as an intravenous bolus to achieve the desired concentration ...
-
3 DOSAGE FORMS AND STRENGTHSEphedrine Sulfate Injection, USP is a clear, colorless, sterile solution for intravenous injection available as : single-dose 1 mL vial that contains 50 mg/mL ephedrine sulfate USP, equivalent to ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Pressor Effect with Concomitant Oxytocic Drugs - Serious postpartum hypertension has been described in patients who received both a vasopressor (i.e., methoxamine, phenylephrine, ephedrine ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of ephedrine sulfate were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain ...
-
7 DRUG INTERACTIONSInteractions that Augment the Pressor Effect - Oxytocin and oxytocic drugs - Clinical Impact: Serious postpartum hypertension has been described in patients who received both a ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from randomized studies, case series, and reports of ephedrine sulfate use in pregnant women have not identified a drug-associated risk of major ...
-
10 OVERDOSAGEOverdose of ephedrine can cause a rapid rise in blood pressure. In the case of an overdose, careful monitoring of blood pressure is recommended. If blood pressure continues to rise to an ...
-
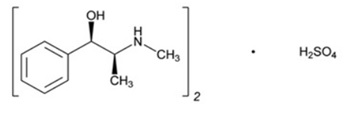
11 DESCRIPTIONEphedrine is an alpha- and beta-adrenergic agonist and a norepinephrine-releasing agent. Ephedrine Sulfate Injection, USP is a clear, colorless, sterile solution for intravenous injection. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ephedrine sulfate is a sympathomimetic amine that directly acts as an agonist at α- and β-adrenergic receptors and indirectly causes the release of norepinephrine from ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Two-year feeding studies in rats and mice conducted under the National Toxicology Program (NTP) demonstrated no ...
-
14 CLINICAL STUDIESThe evidence for the efficacy of ephedrine injection is derived from the published literature. Increases in blood pressure following administration of ephedrine were observed in 14 studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEphedrine Sulfate Injection, USP is a clear, colorless, sterile solution for intravenous injection supplied as follows: NDC - Strength - How Supplied - 65145-158-25 - 50 mg/mL of ...
-
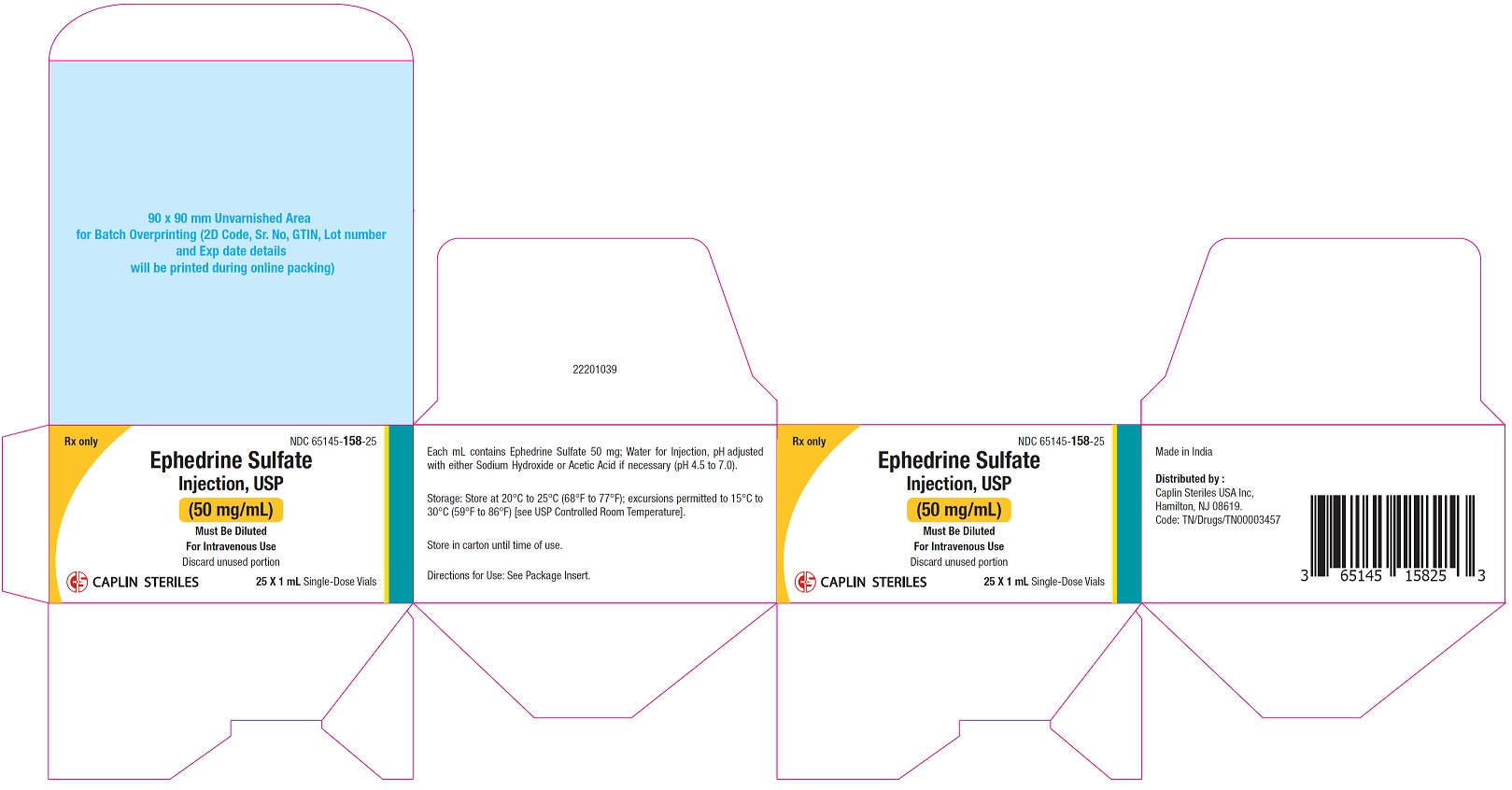
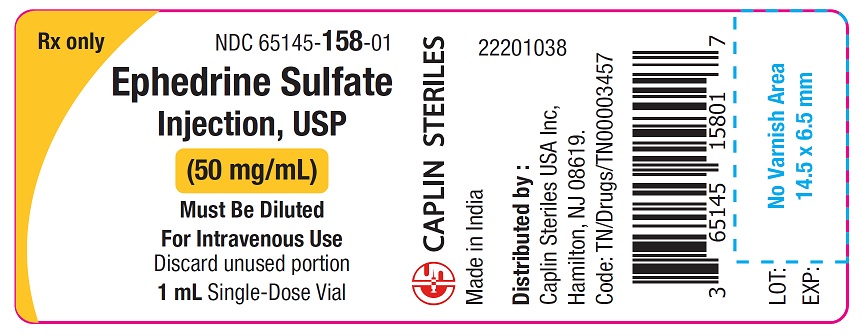
PACKAGE LABEL.PRINCIPAL DISPLAY PANELCarton Label - 50 mg/mL - NDC 65145-158-25 - Rx only - Ephedrine Sulfate - Injection, USP - 50 mg/mL - Must Be Diluted - For Intravenous Use - 25 X 1 mL Single Dose Vials - Container Label - 50 ...
-
INGREDIENTS AND APPEARANCEProduct Information