Label: EPSOM SALT granule
- NDC Code(s): 70677-0038-1, 70677-0038-2
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
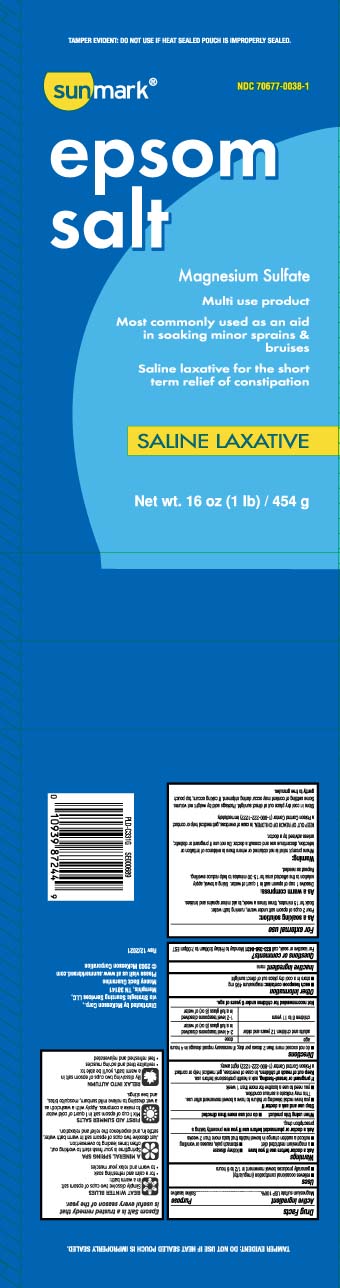
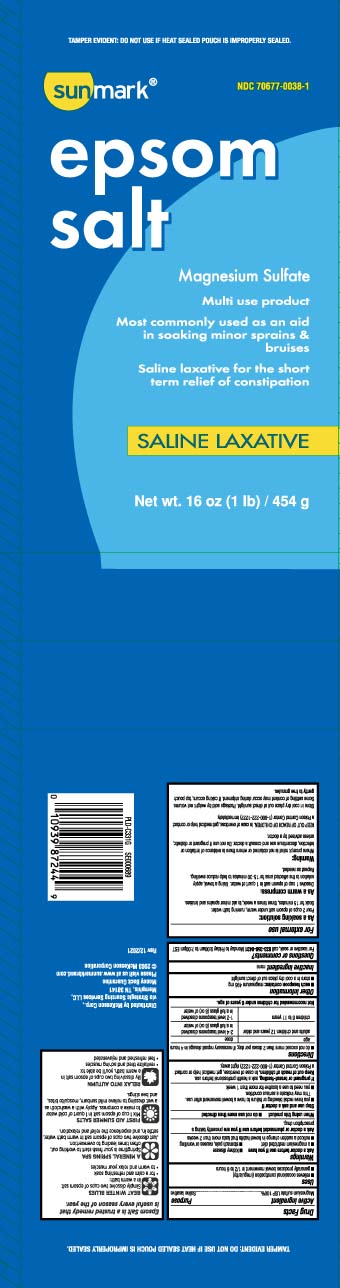
Active ingredientMagnesium sulfate USP 100%
-
PurposeSaline laxative
-
Usesrelieves occasional constipation (irregularity) generally produces bowel movement in 1/2 to 6 hours
-
WarningsAsk a doctor before use if you have - kidney disease - a magnesium restricted diet - stomach pain, nausea or vomiting - noticed a sudden change in bowel habits that lasts more than 2 weeks - Ask ...
-
Directionsdo not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours - agedose - adults and children 12 years and older2-4 level teaspoons dissolved in a full glass (8oz) of ...
-
Other informationeach teaspoon contains: magnesium 495 mg - store in a cool dry place out of direct sunlight
-
Inactive ingredientnone
-
Questions or comments?For laxative or soak, call 1-833-358-6431 Monday- Friday 9AM-7PM EST
-
Principal Display PanelEpsom salt - Magnesium Sulfate - Multi use product - Most commonly used as an aid - in soaking minor sprains & bruises - Saline laxative for the short term relief of constipation - SALINE LAXATIVE - Net Wt LB ...
-
Package LabelSUNMARK Epsom Salt
-
INGREDIENTS AND APPEARANCEProduct Information