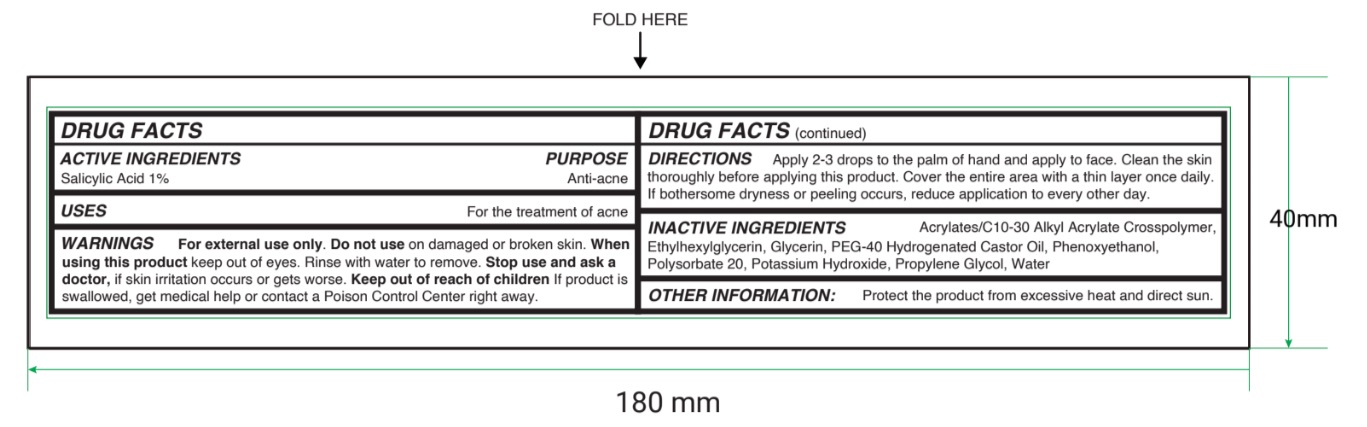
Label: YOUR ANTI ACNE SERUM- salicylic acid liquid
- NDC Code(s): 84229-0016-0
- Packager: Skin Ai, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Other information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
YOUR ANTI ACNE SERUM
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84229-0016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.2 g in 20 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84229-0016-0 20 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 04/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 04/02/2024 Labeler - Skin Ai, LLC (003716972) Registrant - Columbia Cosmetics Manufacturing, Inc. (068267863) Establishment Name Address ID/FEI Business Operations Columbia Cosmetics Manufacturing, Inc. 068267863 manufacture(84229-0016)