Label: ALLERGY RELIEF- diphenhydramine hcl tablet, film coated
- NDC Code(s): 49035-929-12, 49035-929-51, 49035-929-98
- Packager: Wal-Mart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
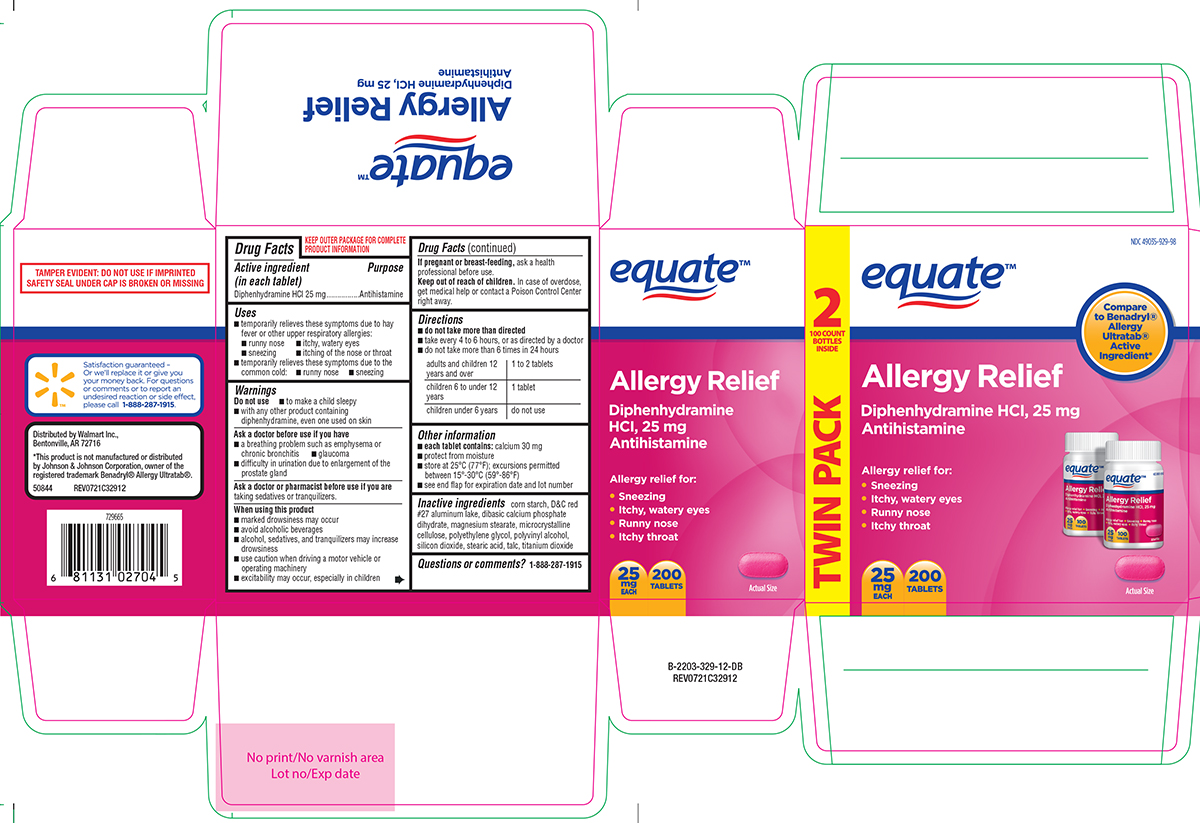
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
-
Purpose
Antihistamine
-
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - itchy, watery eyes - sneezing - itching of the nose or throat - temporarily relieves these ...
-
Warnings
Do not use - to make a child sleepy - with any other product containing diphenhydramine, even one used on skin - Ask a doctor before use if you have - a breathing problem such as emphysema ...
-
Directions
do not take more than directed - take every 4 to 6 hours, or as directed by a doctor - do not take more than 6 times in 24 hours - adults and children 12 years and over1 to 2 tablets - children ...
-
Other information
each tablet contains: calcium 30 mg - protect from moisture - store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) use by expiration date on package
-
Inactive ingredients
corn starch, D&C red #27 aluminum lake, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid ...
-
Questions or comments?
1-888-287-1915
-
Principal Display Panel
equate™ NDC 49035-929-51 - Compare - to Benadryl® Allergy - Ultratabs® Active - Ingredient* Allergy Relief - Diphenhydramine HCl, 25 mg - Antihistamine - Relieves: • Sneezing - • Itchy, watery ...
-
INGREDIENTS AND APPEARANCEProduct Information