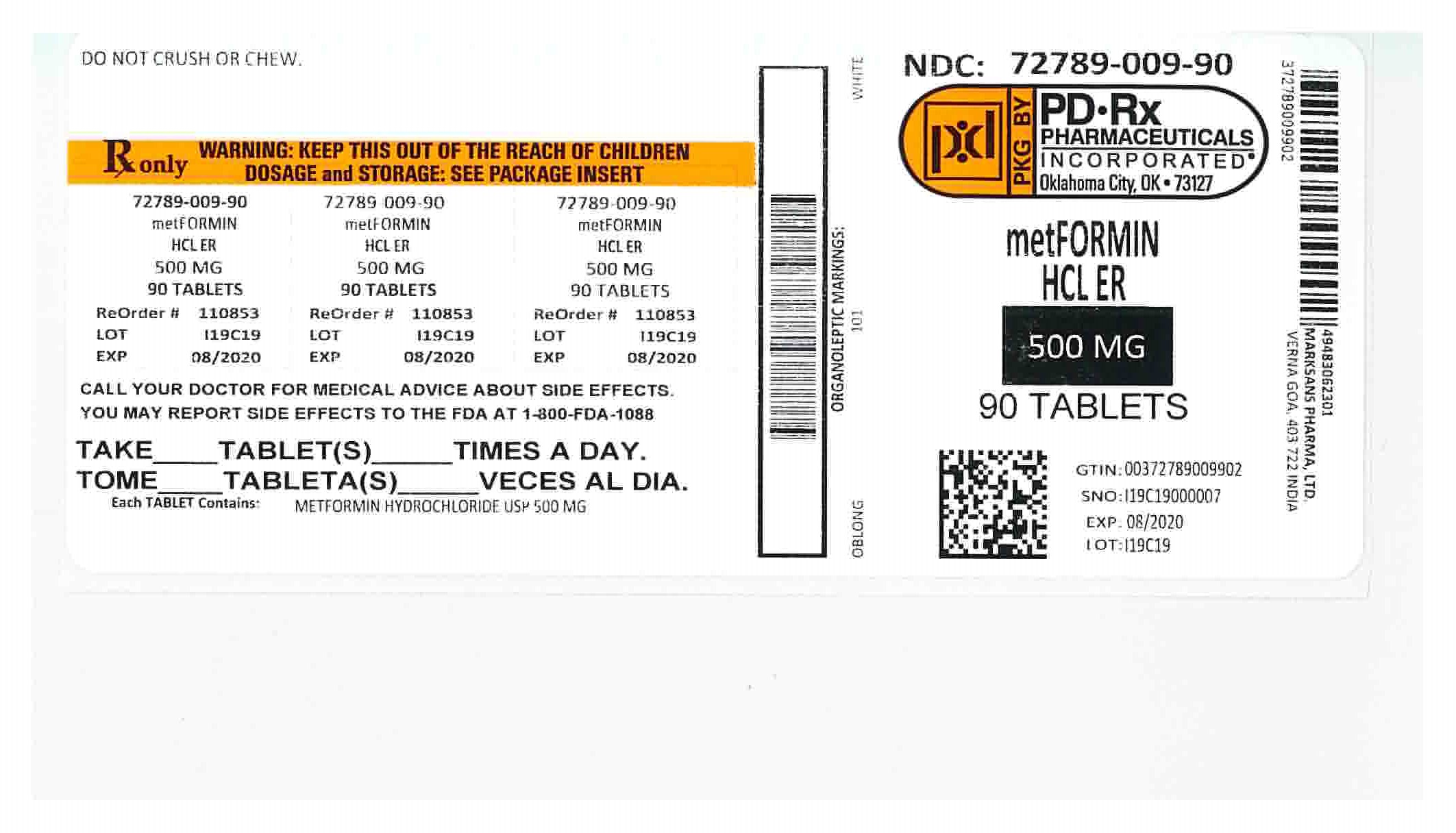
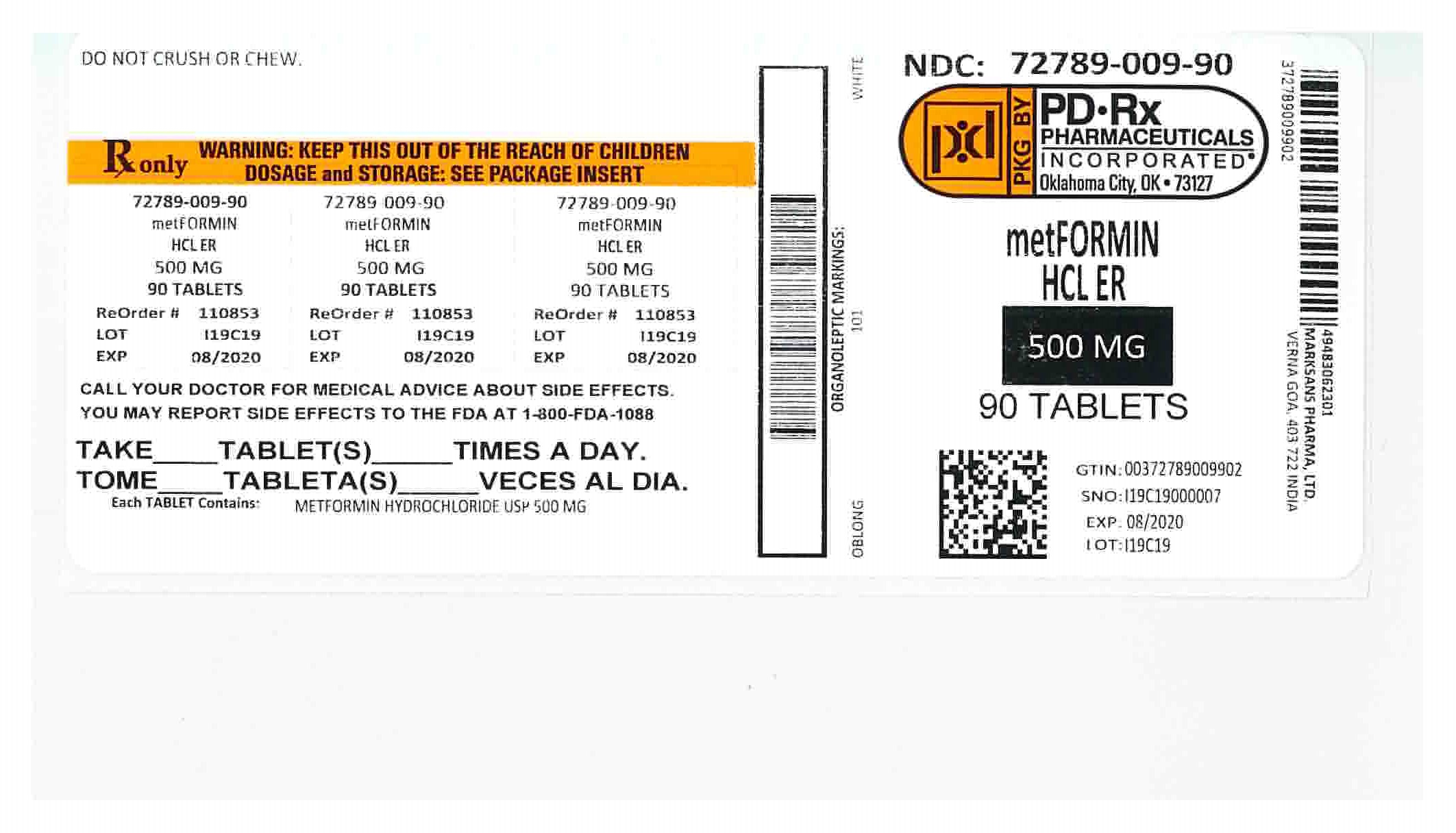
Metformin Hydrochloride Extended-Release Tablets
-
(met for' min hye '' droe klor' ide )
Rx only
-
Read the Patient Information that comes with Metformin Hydrochloride Extended-Release ...
Metformin Hydrochloride Extended-Release Tablets
(met for' min hye '' droe klor' ide )
Rx only
Read the Patient Information that comes with Metformin Hydrochloride Extended-Release Tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know aboutMetformin Hydrochloride Extended-Release Tablets?
Serious side effects can happen in people takingMetformin Hydrochloride Extended-Release Tabletsincluding:
Lactic Acidosis.Metformin hydrochloride, the medicine in Metformin Hydrochloride Extended-Release Tablets, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop takingMetformin Hydrochloride Extended-Release Tabletsand call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
- feel very weak and tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unusual sleepiness or sleep longer than usual
- have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis if you:
- have kidney problems. People whose kidneys are not working properly should not take Metformin Hydrochloride Extended-Release Tablets.
- have liver problems.
- have congestive heart failure that requires treatment with medicines.
- drink a lot of alcohol (very often or short-term “binge” drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery.
- have a heart attack, severe infection, or stroke.
- are 80 years of age or older and have not had your kidney function tested.
What are Metformin Hydrochloride Extended-Release Tablets?
- Metformin Hydrochloride Extended-Release Tablets are prescription medicines that contain metformin hydrochloride. Metformin Hydrochloride Extended-Release Tablets are used with diet and exercise to help control high blood sugar (hyperglycemia) in adults with type 2 diabetes.
- Metformin Hydrochloride Extended-Release Tablets are not for people with type 1 diabetes.
- Metformin Hydrochloride Extended-Release Tablets are not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
Metformin Hydrochloride and Metformin Hydrochloride Extended-Release Tablets have the same active ingredient. However, Metformin Hydrochloride Extended-Release Tablets work longer in your body. Both of these medicines help control your blood sugar in a number of ways.
These include helping your body respond better to the insulin it makes naturally, decreasing the amount of sugar your liver makes, and decreasing the amount of sugar your intestines absorb. Metformin hydrochloride extended-release tablets do not cause your body to make more insulin.
Who should not take Metformin Hydrochloride Extended-Release Tablets?
Some conditions increase your chance of getting lactic acidosis or cause other problems if you take these medicines. Most of the conditions listed below can increase your chance of getting lactic acidosis.
Do not take Metformin Hydrochloride Extended-Release Tabletsif you:
- have kidney problems
- are allergic to the metformin hydrochloride in metformin hydrochloride extended-release tablets or any of the ingredients in metformin hydrochloride extended-release tablets. See the end of this leaflet for a complete list of ingredients in metformin hydrochloride extended-release tablets.
- are going to get an injection of dye or contrast agents for an x-ray procedure or if you are going to have surgery and not able to eat or drink much. In these situations, metformin hydrochloride extended-release tablets will need to be stopped for a short time. Talk to your healthcare provider about when you should stop metformin hydrochloride extended-release tablets and when you should start Metformin Hydrochloride Extended-Release Tablets again. See “
What is the most important information I should know aboutMetformin Hydrochloride Extended-Release Tablets?”
-
What should I tell my healthcare provider before taking Metformin Hydrochloride Extended-Release Tablets?
Before taking metformin hydrochloride extended-release tablets, tell your healthcare provider if you:
- have type 1 diabetes. Metformin hydrochloride extended-release tablets should not be used to treat people with type 1 diabetes.
- have a history or risk for diabetic ketoacidosis (high levels of certain acids, known as ketones, in the blood or urine). Metformin hydrochloride extended-release tablets should not be used for the treatment of diabetic ketoacidosis.
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are older than 80 years. If you are over 80 years old you should not take metformin hydrochloride extended-release tablets unless your kidneys have been checked and they are normal.
- drink alcohol very often or drink a lot of alcohol in short-term “binge” drinking.
- are taking insulin.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if metformin hydrochloride extended-release tablets will harm your unborn baby. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are breast-feeding or plan to breast-feed. It is not known if metformin hydrochloride extended-release tablets passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you take metformin hydrochloride extended-release tablets.
Tell your healthcare provider about all the medicines you take,including prescription and nonprescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
- Metformin hydrochloride extended-release tablets may affect the way other medicines work, and other medicines may affect how metformin hydrochloride extended-release tablets works.
CanMetformin Hydrochloride Extended-Release Tablets be used in children?
Metformin hydrochloride extended-release tablets has not been studied in children.
How should I takeMetformin Hydrochloride Extended-Release Tablets?
- Take metformin hydrochloride extended-release tablets exactly as your healthcare provider tells you.
- Metformin hydrochloride extended-release tablets should be taken with meals to help lessen an upset stomach side effect.
- Swallow metformin hydrochloride extended-release tablets whole. Do not crush, cut, or chew metformin hydrochloride extended-release tablets.
- You may sometimes pass a soft mass in your stools (bowel movement) that looks like metformin hydrochloride extended-release tablets. This is not harmful and will not affect the way metformin hydrochloride extended-release tablets works to control your diabetes.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your healthcare provider right away if you have any of these problems.
- Your healthcare provider should do blood tests to check how well your kidneys are working before and during your treatment with metformin hydrochloride extended-release tablets.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your haemoglobin A1C.
- Follow your healthcare provider’s instructions for treating blood sugar that is too low (hypoglycemia). Talk to your healthcare provider if low blood sugar is a problem for you. See
“What are the possible side effects ofMetformin Hydrochloride Extended-Release Tablets?”
- Check your blood sugar as your healthcare provider tells you to.
- Stay on your prescribed diet and exercise program while taking metformin hydrochloride extended-release tablets.
- If you miss a dose of metformin hydrochloride extended-release tablets, take your next dose as prescribed unless your healthcare provider tells you differently. Do not take an extra dose the next day.
- If you take too much metformin hydrochloride extended-release tablets, call your healthcare provider, local Poison Control Center, or go to the nearest hospital emergency room right away.
What should I avoid while takingMetformin Hydrochloride Extended-Release Tablets?
Do not drink a lot of alcoholic drinks while taking metformin hydrochloride extended-release tablets. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Alcohol can increase the chance of getting lactic acidosis.
What are the side effects ofMetformin Hydrochloride Extended-Release Tablets?
-
Lactic Acidosis.Metformin, the active ingredient inMetformin Hydrochloride Extended-Release Tablets, can cause a rare but serious condition called lactic acidosis (a buildup of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Call your doctor right away if you have any of the following symptoms, which could be signs of lactic acidosis:
- you feel cold in your hands or feet
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
- you feel very weak or tired
- you have trouble breathing
- you feel sleepy or drowsy
- you have stomach pains, nausea or vomiting
Most people who have had lactic acidosis with metformin have other things that, combined with the metformin, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with metformin hydrochloride extended-release tablet if you:
- have severe kidney problems, or your kidneys are affected by certain x-ray tests that use injectable dye
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids
- have surgery
- have a heart attack, severe infection, or stroke
Common side effects of metformin hydrochloride extended-release tablets include diarrhea, nausea, and upset stomach. These side effects generally go away after you take the medicine for a while. Taking your medicine with meals can help reduce these side effects. Tell your doctor if the side effects bother you a lot, last for more than a few weeks, come back after they’ve gone away, or start later in therapy. You may need a lower dose or need to stop taking the medicine for a short period or for good.
About 3 out of every 100 people who take metformin hydrochloride extended-release tablets have an unpleasant metallic taste when they start taking the medicine. It lasts for a short time.
Metformin hydrochloride extended-release tablets rarely cause hypoglycemia (low blood sugar) by themselves. However, hypoglycemia can happen if you do not eat enough, if you drink alcohol, or if you take other medicines to lower blood sugar.
How should I storeMetformin Hydrochloride Extended-Release Tablets?
Store metformin hydrochloride extended-release tablets at 68°F to 77°F (20°C to 25°C).
Keepmetformin hydrochloride extended-release tabletsand all medicines out of the reach of children.
General information about the use ofmetformin hydrochloride extended-release tablets
If you have questions or problems, talk with your doctor or other healthcare provider. You can ask your doctor or pharmacist for the information about metformin hydrochloride extended-release tablets that is written for health care professionals. Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use metformin hydrochloride extended-release tablets for a condition for which it was not prescribed. Do not share your medicine with other people.
What are the ingredients ofMetformin Hydrochloride Extended-Release Tablets?
Active ingredient of metformin hydrochloride extended-release tablets: metformin hydrochloride
Metformin hydrochloride extended-release tablets, 500 mg or 750 mg contains the inactive ingredients colloidal silicon dioxide, hypromellose and magnesium stearate.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level.
High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Talk to your healthcare provider about how to prevent, recognize, and take care of low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and problems you have because of your diabetes.
Manufactured for:
Time-Cap Labs, Inc.
7 Michael Avenue, Farmingdale, NY 11735, USA
Manufactured by:
Marksans Pharma Ltd.
Plot No.L-82, L-83, Vema Indl. Estate,
Vena, Goa-403 722, India
Rev. 06/23
Close