Label: POTASSIUM CHLORIDE solution
- NDC Code(s): 64950-320-47, 64950-320-99, 64950-322-47
- Packager: Genus Lifesciences
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CHLORIDE safely and effectively. See full prescribing information for POTASSIUM CHLORIDE. POTASSIUM CHLORIDE oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPotassium Chloride is indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration and Monitoring - Monitoring - Monitor serum potassium and adjust dosages accordingly. For treatment of hypokalemia, monitor potassium levels daily or more often depending on ...
-
3 DOSAGE FORMS AND STRENGTHSOral Solution 10%: 1.3 mEq potassium per mL. Oral Solution 20%: 2.6 mEq potassium per mL.
-
4 CONTRAINDICATIONSPotassium chloride is contraindicated in patients on potassium sparing diuretics.
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Irritation - May cause gastrointestinal irritation if administered undiluted. Increased dilution of the solution and taking with meals may reduce gastrointestinal irritation ...
-
6 ADVERSE REACTIONSThe most common adverse reactions to oral potassium salts are nausea, vomiting, flatulence, abdominal pain/discomfort, and diarrhea.
-
7 DRUG INTERACTIONS7.1 Potassium-Sparing Diuretics - Use with potassium-sparing diuretics can produce severe hyperkalemia. Avoid concomitant use. 7.2 Renin-Angiotensin-Aldosterone System Inhibitors - Drugs that ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There are no human data related to use of Potassium Chloride during pregnancy, and animal studies have not been conducted. Potassium supplementation that does not lead to ...
-
10 OVERDOSAGE10.1 Symptoms - The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are ...
-
11 DESCRIPTIONPotassium Chloride is a white crystalline or colorless solid. It is soluble in water and slightly soluble in alcohol. Chemically, Potassium Chloride is K-Cl with a molecular mass of 74.55. Oral ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The potassium ion (K+) is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including ...
-
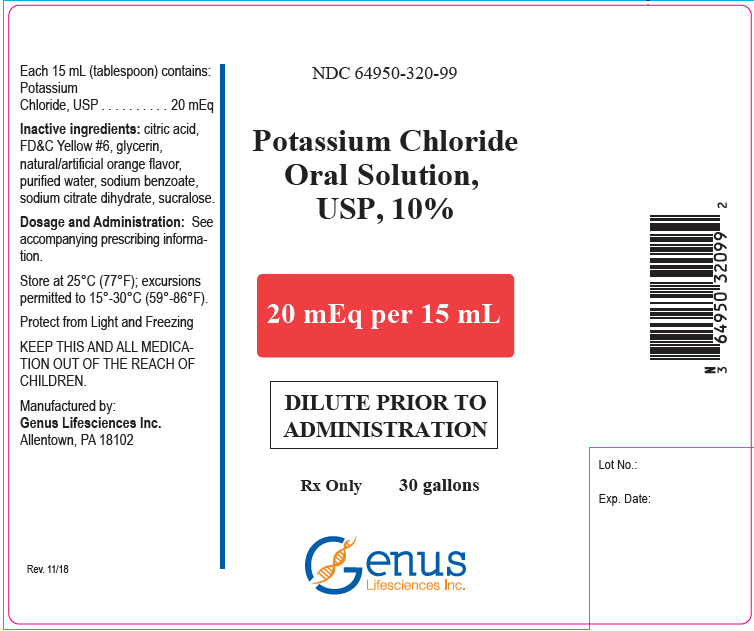
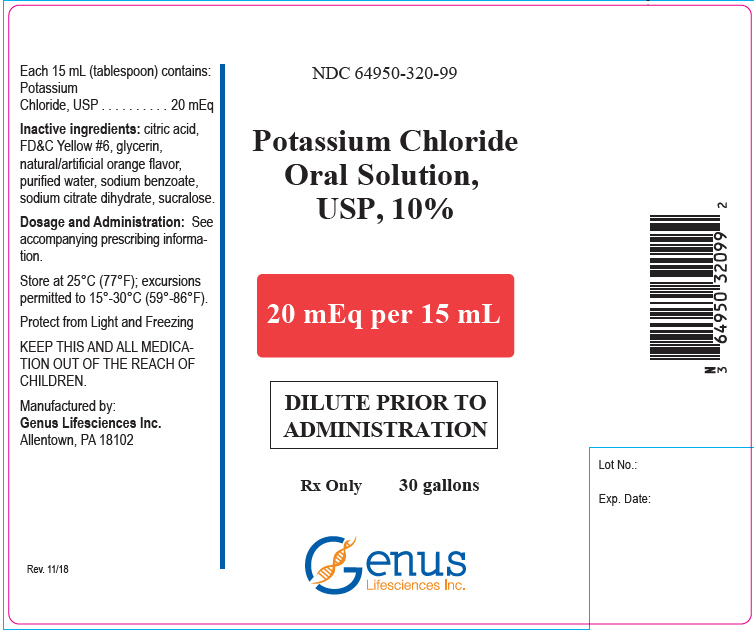
16 HOW SUPPLIED/STORAGE AND HANDLINGPotassium Chloride Oral Solution, is an orange solution available in two strengths as follows: 10%: 20 mEq/15 mL oral solution - NDC# 64950-320-47 Bottle of 473 mL - 20%: 40 mEq/15 mL oral ...
-
SPL UNCLASSIFIED SECTIONRx only - Manufactured by: Genus Lifesciences Inc. Allentown, PA 18102 - Revised: 9/2024
-
PRINCIPAL DISPLAY PANEL - 20 mEq Bottle LabelNDC 64950-320-47 - Potassium Chloride - Oral Solution, USP, 10% 20 mEq per 15 mL - DILUTE PRIOR TO - ADMINISTRATION - Rx Only - 473 mL - Genus - Lifesciences Inc.
-
PRINCIPAL DISPLAY PANEL - 40 mEq Bottle LabelNDC 64950-322-47 - Potassium Chloride - Oral Solution, USP, 20% 40 mEq per 15 mL - DILUTE PRIOR TO - ADMINISTRATION - Rx Only - 473 mL - Genus - Lifesciences Inc.
-
INGREDIENTS AND APPEARANCEProduct Information