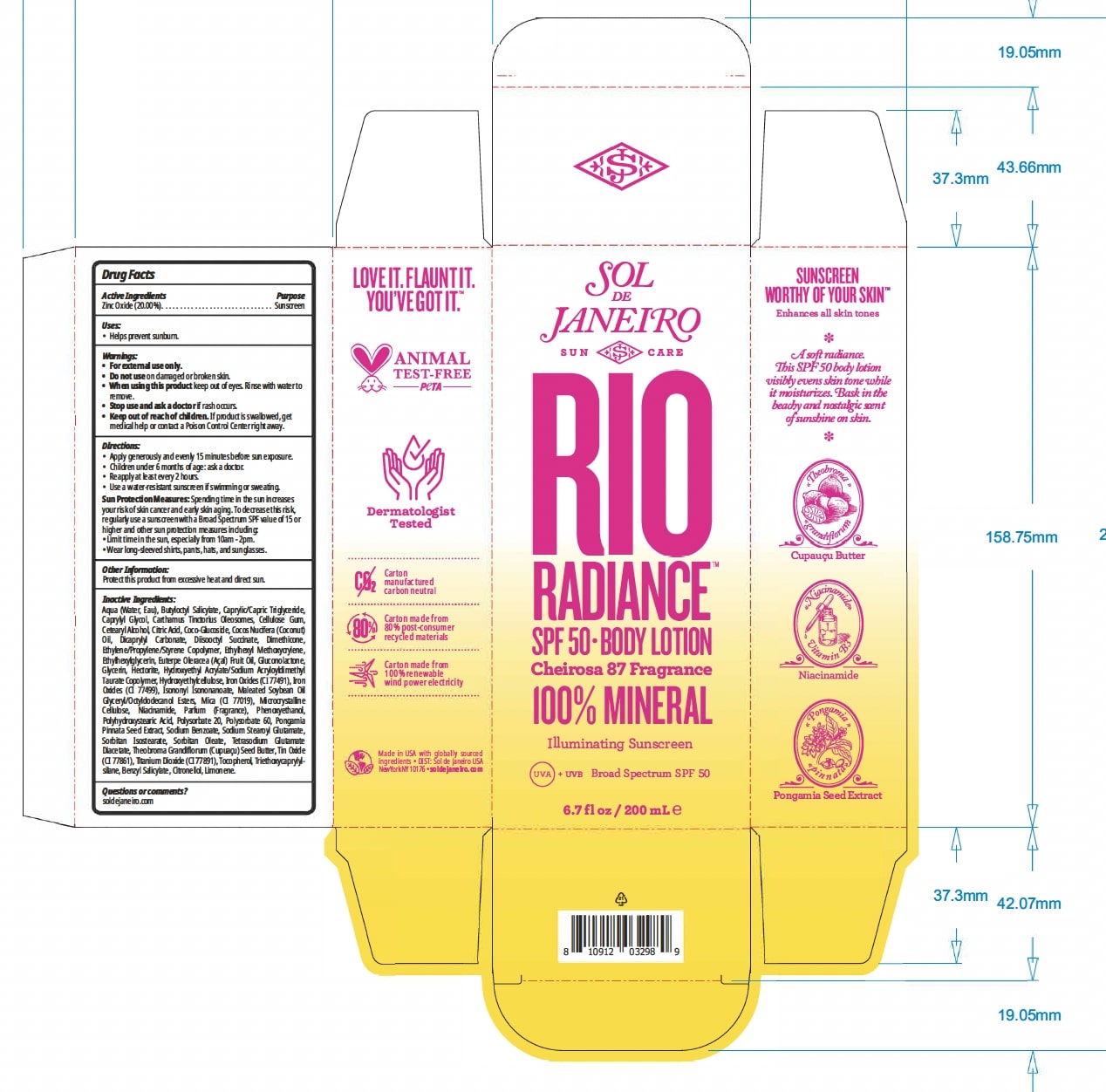
Label: RIO RADIANCE SPF 50 BODYLOTION- zinc oxide lotion
- NDC Code(s): 83982-4838-0
- Packager: SOL DE JANEIRO USA, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warnings:
• For external use only.
• Do not use on damaged or broken skin.
• When using this product keep out of eyes. Rinse with water to
remove.
• Stop use and ask a doctor if rash occurs.
• Keep out of reach of children. If product is swallowed, get
medical help or contact a Poison Control Center right away.Sun Protection Measures: Spending time in the sun increases
your risk of skin cancer and early skin aging. To decrease this risk,
regularly use a sunscreen with a Broad Spectrum SPF value of 15 or
higher and other sun protection measures including:
• Limit time in the sun, especially from 10am - 2pm.
• Wear long-sleeved shirts, pants, hats, and sunglasses.
-
INACTIVE INGREDIENT
Aqua (Water, Eau), Butyloctyl Salicylate, Caprylic/Capric Triglyceride, Caprylyl Glycol, Carthamus Tinctorius Oleosomes, Cellulose Gum, Cetearyl Alcohol, Citric Acid, Coco-Glucoside, Cocos Nucifera (Coconut) Oil, Dicaprylyl Carbonate, Diisooctyl Succinate, Dimethicone, Ethylene/Propylene/Styrene Copolymer, Ethylhexyl Methoxycrylene, Ethylhexylglycerin, Euterpe Oleracea (Açaí) Fruit Oil, Gluconolactone, Glycerin, Hectorite, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydroxyethylcellulose, Iron Oxides (CI 77491), Iron Oxides (CI 77499), Isononyl Isononanoate, Maleated Soybean Oil Glyceryl/Octyldodecanol Esters, Mica (CI 77019), Microcrystalline Cellulose, Niacinamide, Parfum (Fragrance), Phenoxyethanol, Polyhydroxystearic Acid, Polysorbate 20, Polysorbate 60, Pongamia Pinnata Seed Extract, Sodium Benzoate, Sodium Stearoyl Glutamate, Sorbitan Isostearate, Sorbitan Oleate, Tetrasodium Glutamate Diacetate, Theobroma Grandiflorum (Cupuaçu) Seed Butter, Tin Oxide (CI 77861), Titanium Dioxide (CI 77891), Tocopherol, Triethoxycaprylylsilane, Benzyl Salicylate, Citronellol, Limonene.
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIO RADIANCE SPF 50 BODYLOTION
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83982-4838 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 20 g in 100 mL Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NIACINAMIDE (UNII: 25X51I8RD4) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) HECTORITE (UNII: 08X4KI73EZ) SAFFLOWER OIL (UNII: 65UEH262IS) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FERRIC OXIDE RED (UNII: 1K09F3G675) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYSORBATE 60 (UNII: CAL22UVI4M) STANNIC OXIDE (UNII: KM7N50LOS6) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) COCONUT OIL (UNII: Q9L0O73W7L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICA (UNII: V8A1AW0880) TOCOPHEROL (UNII: R0ZB2556P8) THEOBROMA GRANDIFLORUM SEED BUTTER (UNII: I711F13FXM) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) ACAI OIL (UNII: Z0W6766A2W) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) GLUCONOLACTONE (UNII: WQ29KQ9POT) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYSORBATE 20 (UNII: 7T1F30V5YH) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83982-4838-0 1 in 1 CARTON 03/27/2024 1 240 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/27/2024 Labeler - SOL DE JANEIRO USA, INC. (080098027)