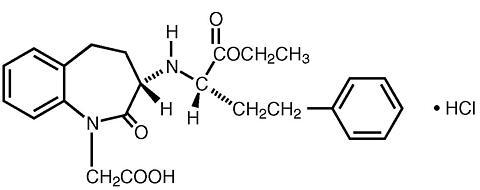
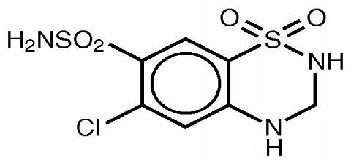
Anaphylactoid and Possibly Related Reactions
-
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin ...
Anaphylactoid and Possibly Related Reactions
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including benazepril) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with angiotensin-converting enzyme inhibitors. In U.S. clinical trials, symptoms consistent with angioedema were seen in none of the subjects who received placebo and in about 0.5% of the subjects who received benazepril. Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, treatment with benazepril hydrochloride and hydrochlorothiazide tablets should be discontinued and appropriate therapy instituted immediately. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine injection 1:1000 (0.3 to 0.5 mL) should be promptly administered (see PRECAUTIONS and ADVERSE REACTIONS).
Black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to nonblacks.
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypersensitivity reactions to hydrochlorothiazide are more likely in patients with allergy and asthma.
Hypotension
Benazepril hydrochloride and hydrochlorothiazide can cause symptomatic hypotension. Like other ACE inhibitors, benazepril has been only rarely associated with hypotension in uncomplicated hypertensive patients. Symptomatic hypotension is most likely to occur in patients who have been volume and/or salt depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume and/or salt depletion should be corrected before initiating therapy with benazepril hydrochloride and hydrochlorothiazide.
Benazepril hydrochloride and hydrochlorothiazide should be used cautiously in patients receiving concomitant therapy with other antihypertensives. The thiazide component of benazepril hydrochloride and hydrochlorothiazide may potentiate the action of other antihypertensive drugs, especially ganglionic or peripheral adrenergic-blocking drugs. The antihypertensive effects of the thiazide component may also be enhanced in the postsympathectomy patient.
In patients with congestive heart failure, with or without associated renal insufficiency, ACE inhibitor therapy may cause excessive hypotension, which may be associated with oliguria, azotemia, and (rarely) with acute renal failure and death. In such patients, benazepril hydrochloride and hydrochlorothiazide tablets therapy should be started under close medical supervision; they should be followed closely for the first 2 weeks of treatment and whenever the dose of benazepril or diuretic is increased.
If hypotension occurs, the patient should be placed in a supine position, and, if necessary, treated with intravenous infusion of physiological saline. Benazepril hydrochloride and hydrochlorothiazide treatment usually can be continued following restoration of blood pressure and volume.
Impaired Renal Function
Monitor renal function periodically in patients treated with benazepril hydrochloride and hydrochlorothiazide. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute of acute renal failure on benazepril hydrochloride and hydrochlorothiazide. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on benazepril hydrochloride and hydrochlorothiazide.
In a small study of hypertensive patients with unilateral or bilateral renal artery stenosis, treatment with benazepril was associated with increases in blood urea nitrogen and serum creatinine; these increases were reversible upon discontinuation of benazepril therapy, concomitant diuretic therapy, or both.
Neutropenia/Agranulocytosis
Another angiotensin-converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients (incidence probably less than once per 10,000 exposures) but more frequently (incidence possibly as great as once per 1000 exposures) in patients with renal impairment, especially those who also have collagen-vascular diseases such as systemic lupus erythematosus or scleroderma. Available data from clinical trials of benazepril are insufficient to show that benazepril does not cause agranulocytosis at similar rates. Monitoring of white blood cell counts should be considered in patients with collagen-vascular disease, especially if the disease is associated with impaired renal function.
Fetal toxicity
Pregnancy category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue benazepril hydrochloride and hydrochlorothiazide as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue benazepril hydrochloride and hydrochlorothiazide, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to benazepril hydrochloride and hydrochlorothiazide for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
No teratogenic effects of benazepril were seen in studies of pregnant rats, mice, and rabbits. On a mg/m2 basis, the doses used in these studies were 60 times (in rats), 9 times (in mice), and more than 0.8 times (in rabbits) the maximum recommended human dose (assuming a 50 kg woman). On a mg/kg basis these multiples are 300 times (in rats), 90 times (in mice), and more than 3 times (in rabbits) the maximum recommended human dose. When hydrochlorothiazide was orally administered without benazepril to pregnant mice and rats during their respective periods of major organogenesis, at doses up to 3000 and 1000 mg/kg/day respectively, there was no evidence of harm to the fetus. Similarly, no teratogenic effects of benazepril were seen in studies of pregnant rats, mice, and rabbits; on a mg/kg basis, the doses used in these studies were 300 times (in rats), 90 times (in mice), and more than 3 times (in rabbits) the maximum recommended human dose.
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma Hydrochlorothiazide, like other diuretics, can cause placental hypoperfusion. It accumulates in the amniotic fluid, with reported concentrations up to 19 times higher than in umbilical vein plasma. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice or thrombocytopenia. Since they do not prevent or alter the course of EPH (Edema, Proteinuria, Hypertension) gestosis (pre-eclampsia), these drugs must not be used to treat hypertension in pregnant women. The use of hydrochlorothiazide for other indications (e.g., heart disease) in pregnancy should be avoided.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Systemic Lupus Erythematosus
Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Close