Label: PENICILLIN G POTASSIUM injection, powder, for solution
- NDC Code(s): 70860-126-20, 70860-126-41, 70860-127-51
- Packager: Athenex Pharmaceutical Division, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONAthenex - Rx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Penicillin G Potassium for Injection, USP and other antibacterial drugs ...
-
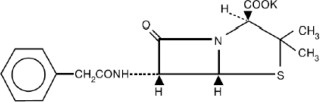
DESCRIPTIONPenicillin G Potassium, USP is a natural penicillin. It is chemically designated 4-Thia-1-azabicyclo [3.2.0]heptane-2-carboxylic acid,3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-, monopotassium ...
-
CLINICAL PHARMACOLOGYAfter an intravenous infusion of penicillin G, peak serum concentrations are attained immediately after completion of the infusion. In a study of ten patients administered a single 5 million unit ...
-
INDICATIONS AND USAGETherapy - Penicillin G Potassium for Injection, USP is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed ...
-
CONTRAINDICATIONSA history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
-
WARNINGSSerious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a ...
-
PRECAUTIONSGeneral - Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma (see - WARNINGS). Whenever allergic reactions occur, penicillin should be ...
-
ADVERSE REACTIONSBody as a whole - The Jarisch-Herxheimer reaction is a systemic reaction, that may occur after the initiation of penicillin therapy in patients with syphilis or other spirochetal infections ...
-
OVERDOSAGEDose related toxicity may arise with the use of massive doses of intravenous penicillins (40 to 100 million units per day), particularly in patients with severe renal impairment (see ...
-
DOSAGE AND ADMINISTRATIONPenicillin G Potassium for Injection, USP may be given intravenously or intramuscularly. The usual dose recommendations are as follows: Adult patients - (*) Because of its short half-life ...
-
HOW SUPPLIEDPenicillin G Potassium for Injection, USP is supplied as follows: NDCPenicillin G Potassium for Injection, USPPackage Factor - 70860-126-205,000,000 units per vial10 vials per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – VIAL LABEL - NDC 70860-126-20 - Penicillin G Potassium for Injection, USP - 5,000,000 units per vial (5 million units per vial) For Intramuscular or Intravenous ...
-
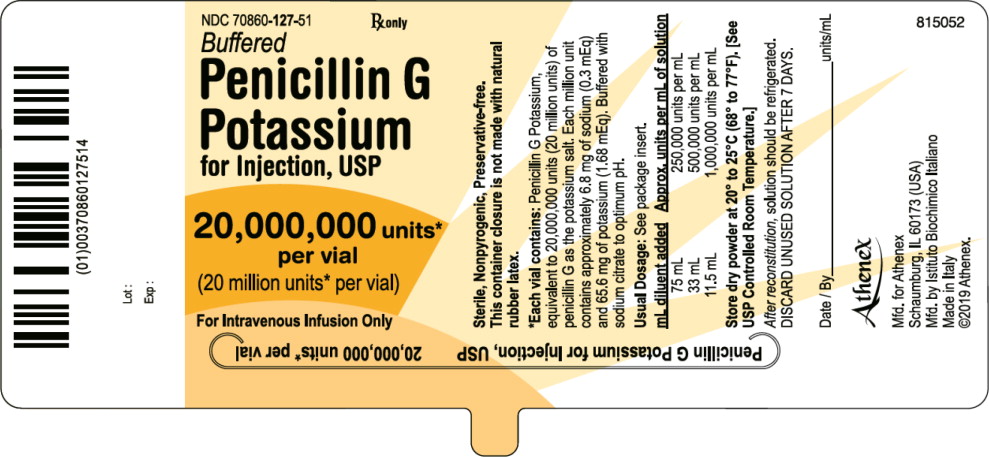
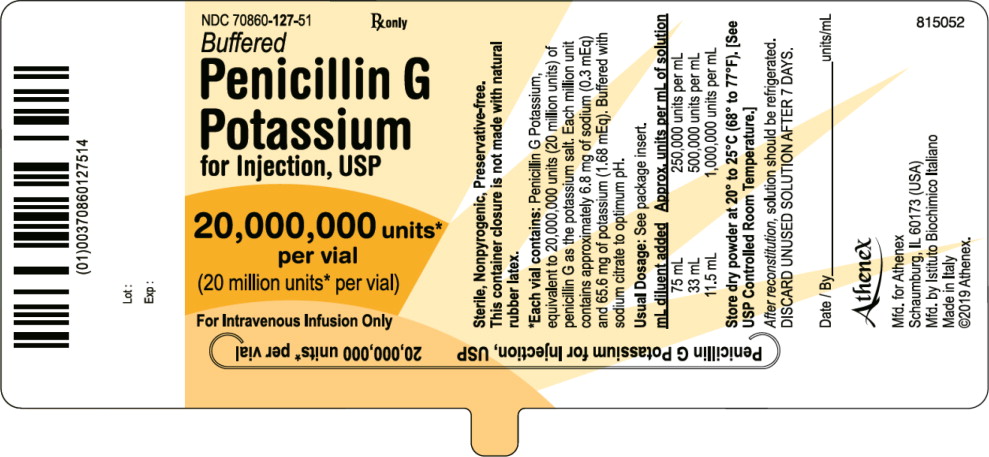
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – VIAL LABEL - NDC 70860-127-51 - Penicillin G Potassium for Injection, USP - 20,000,000 units per vial (20 million units per vial) For Intravenous Infusion ...
-
INGREDIENTS AND APPEARANCEProduct Information