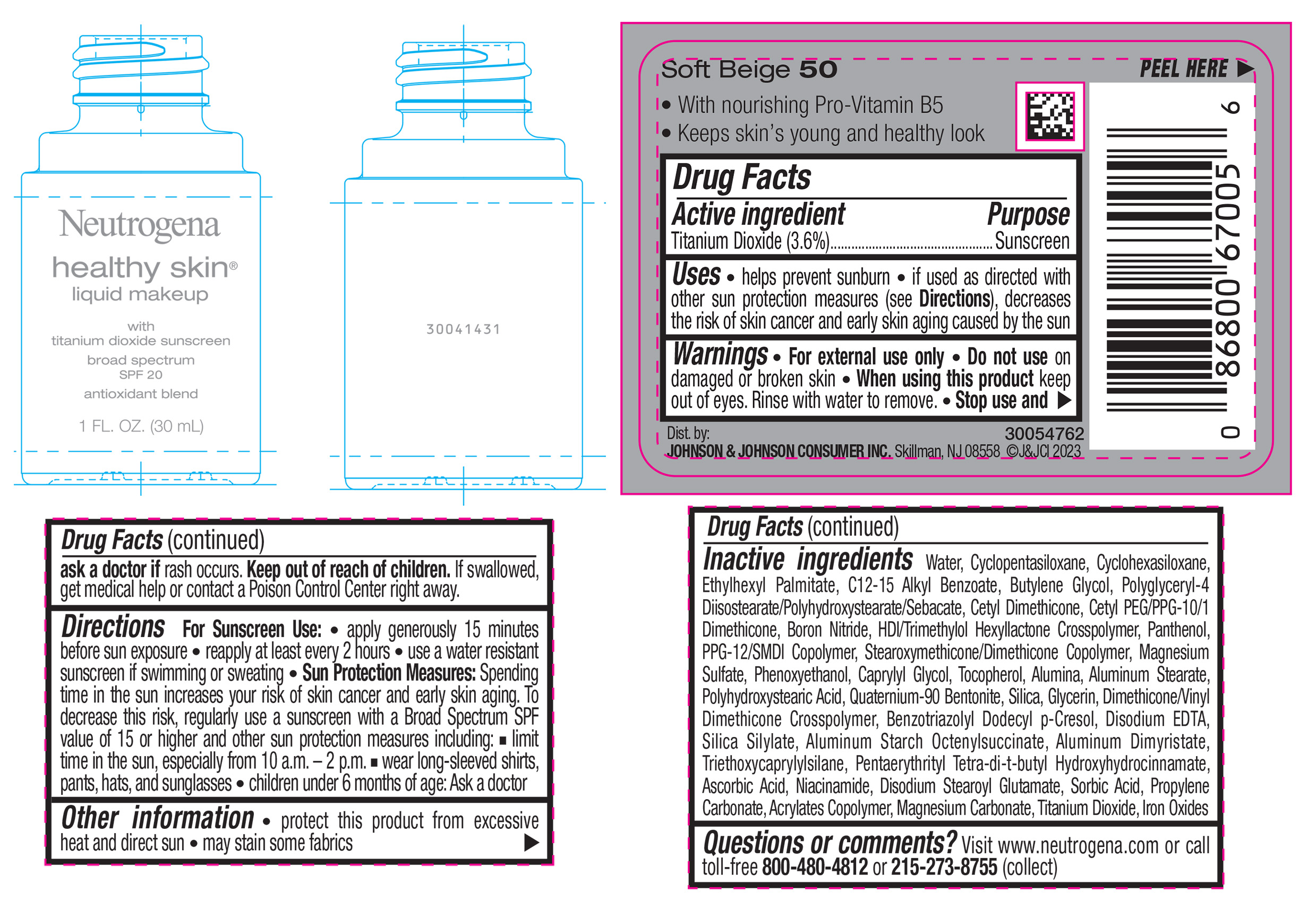
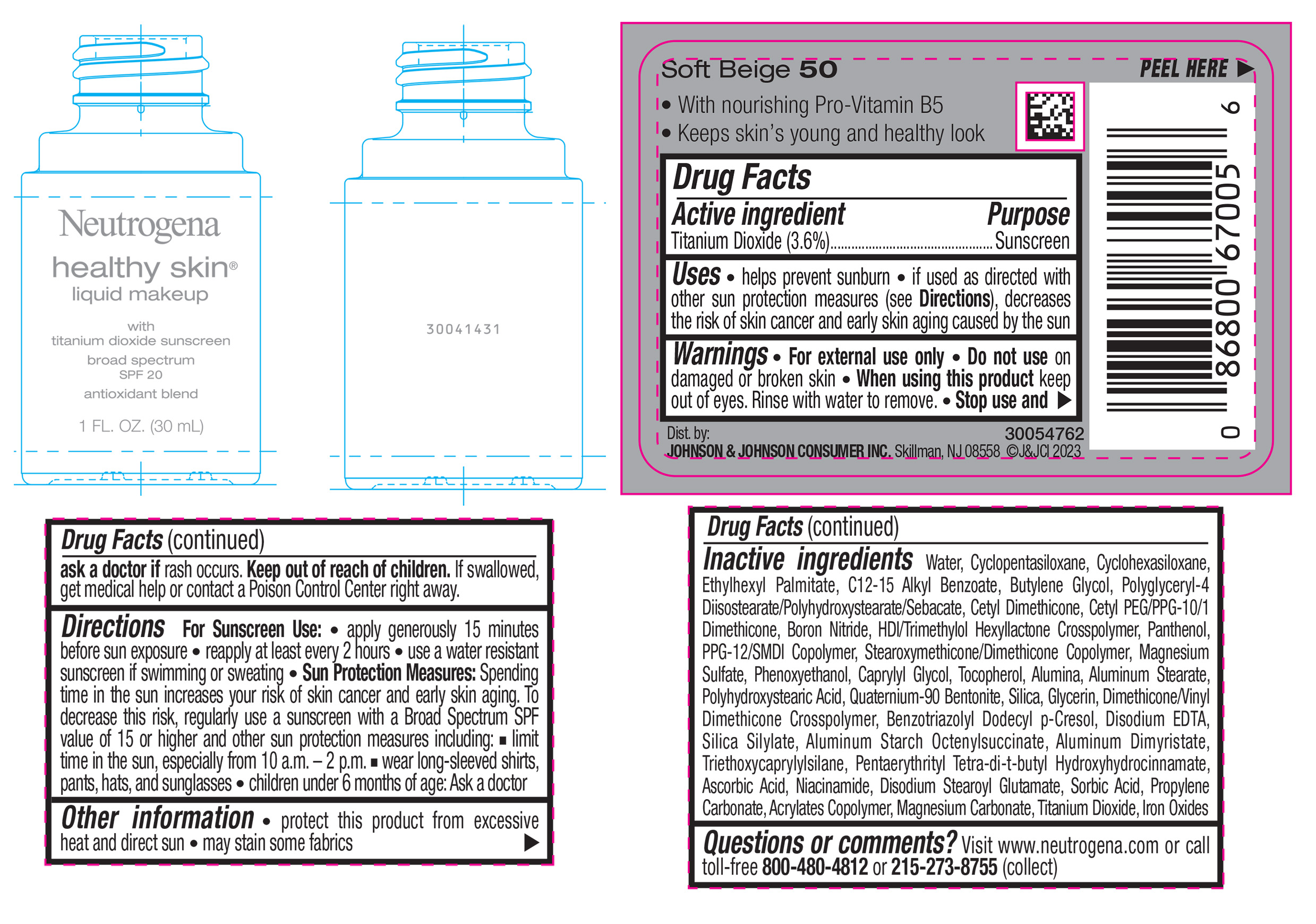
Label: NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - SOFT BEIGE 50- titanium dioxide liquid
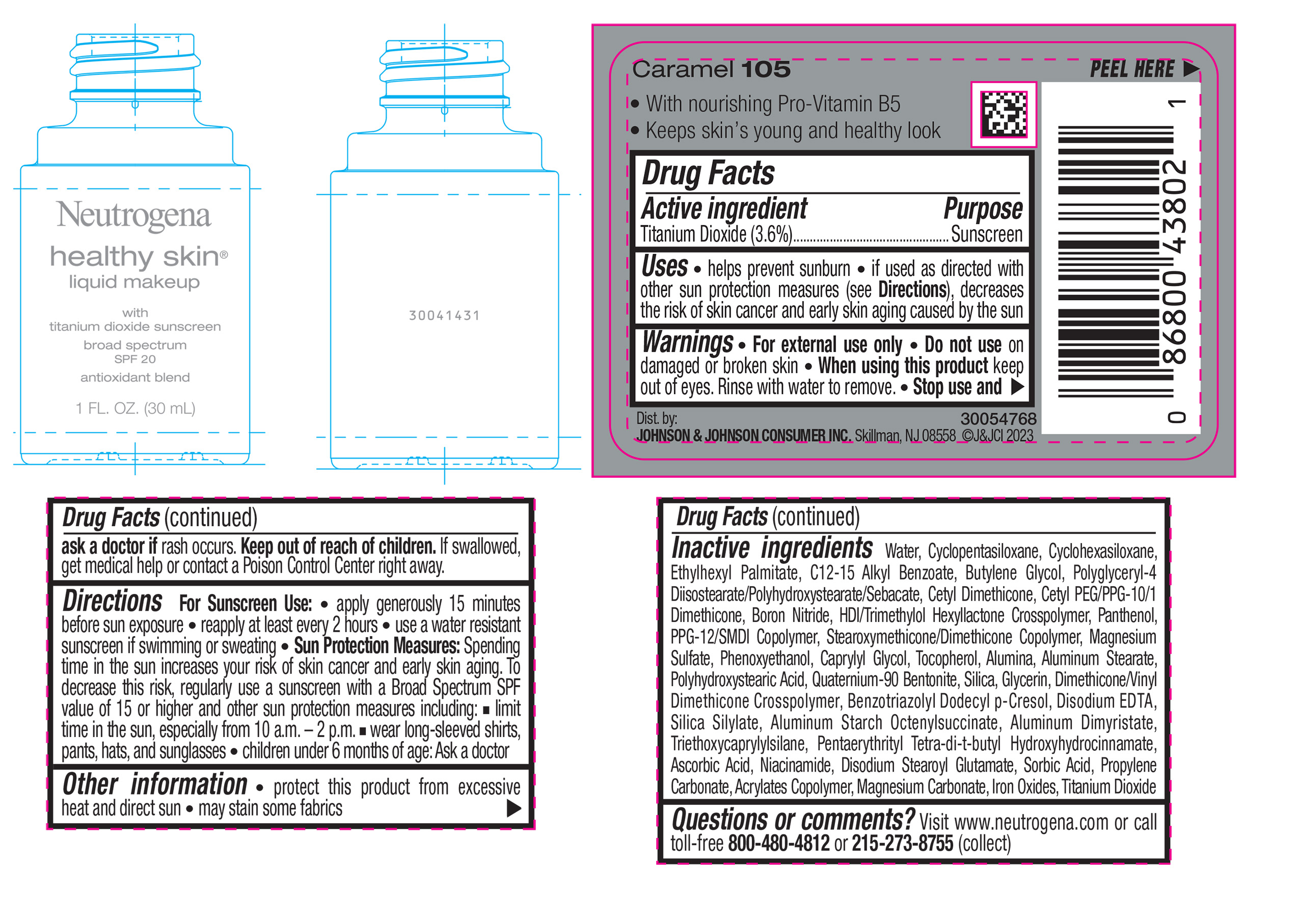
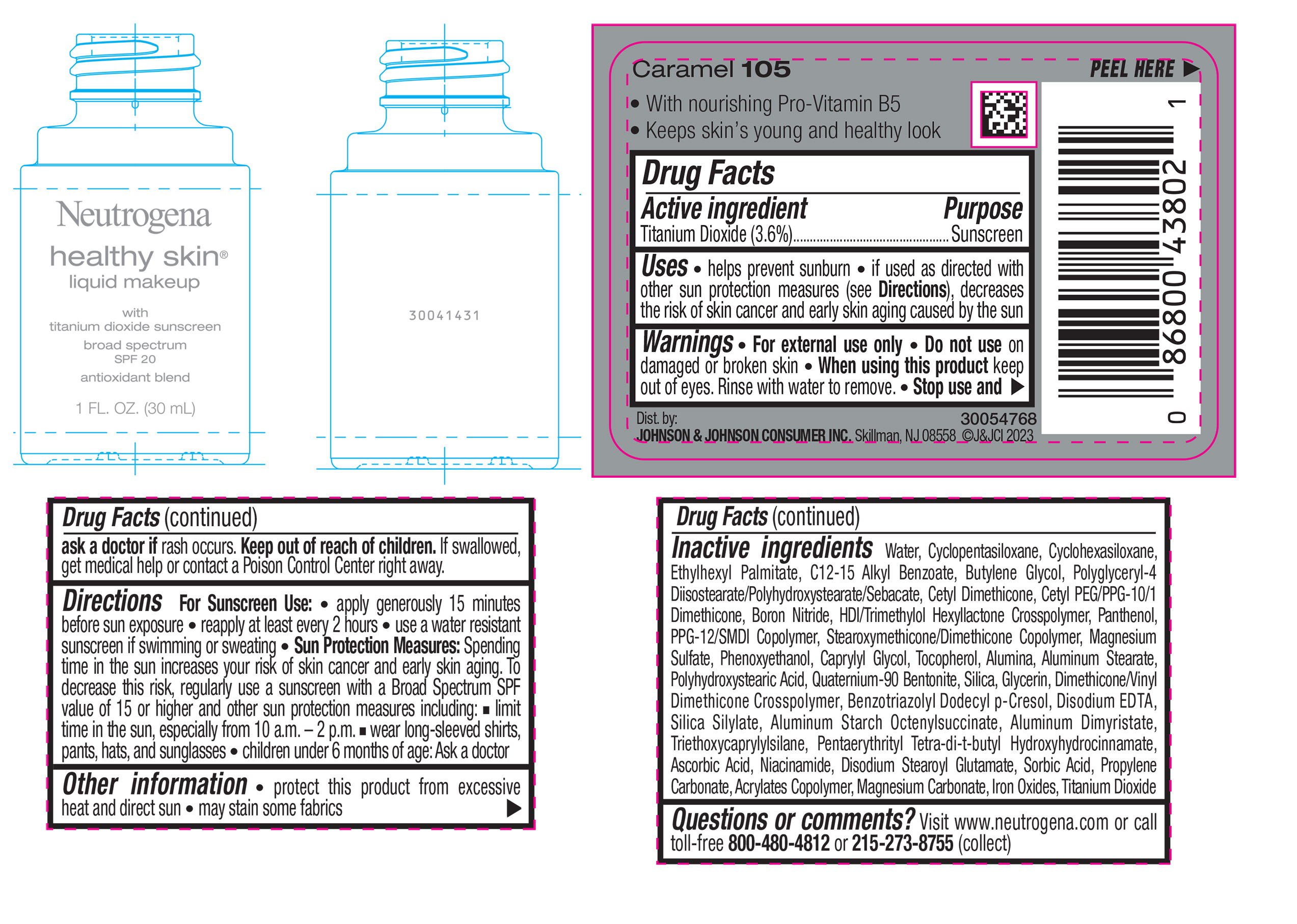
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CARAMEL 105- titanium dioxide liquid
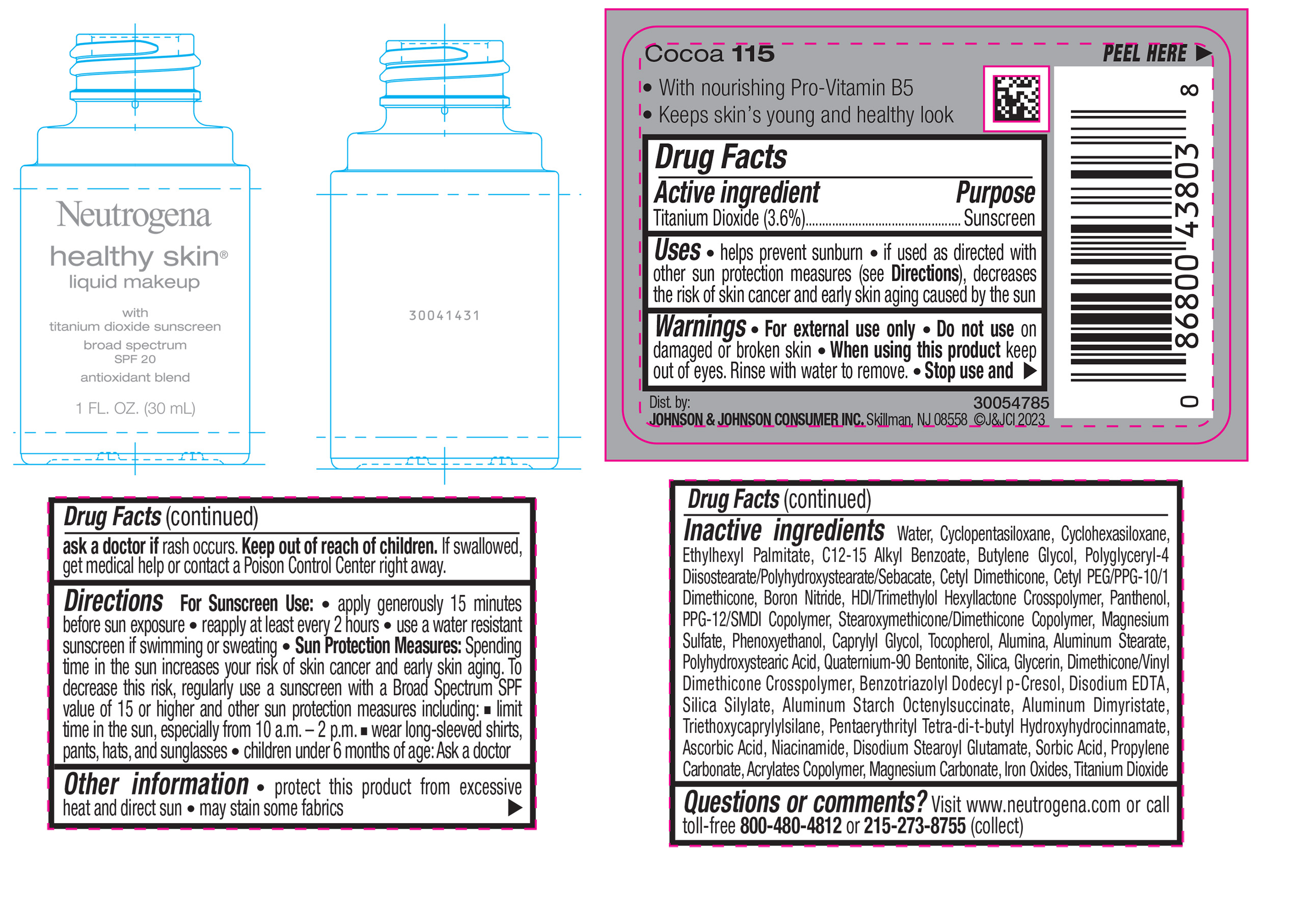
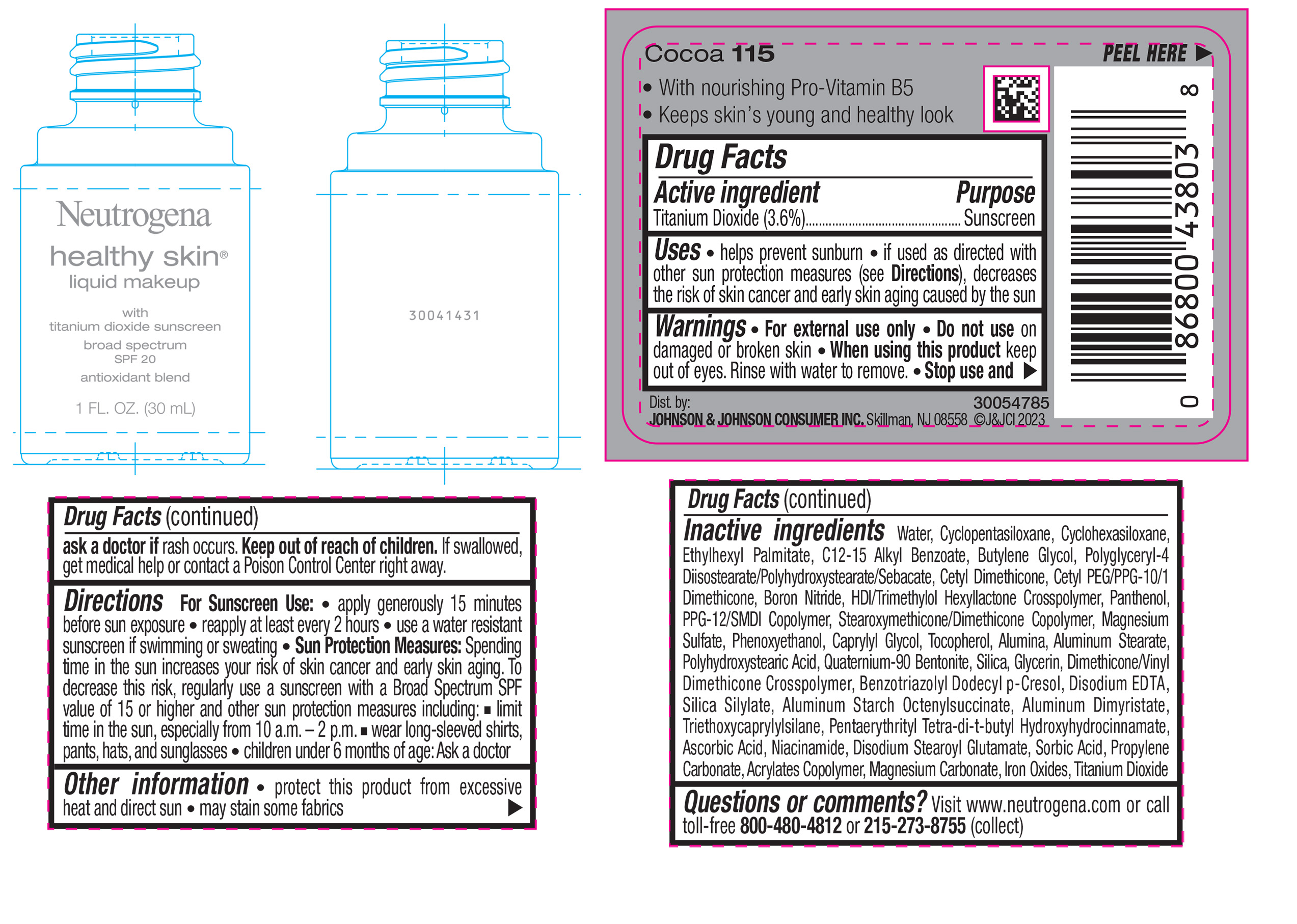
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - COCOA 115- titanium dioxide liquid
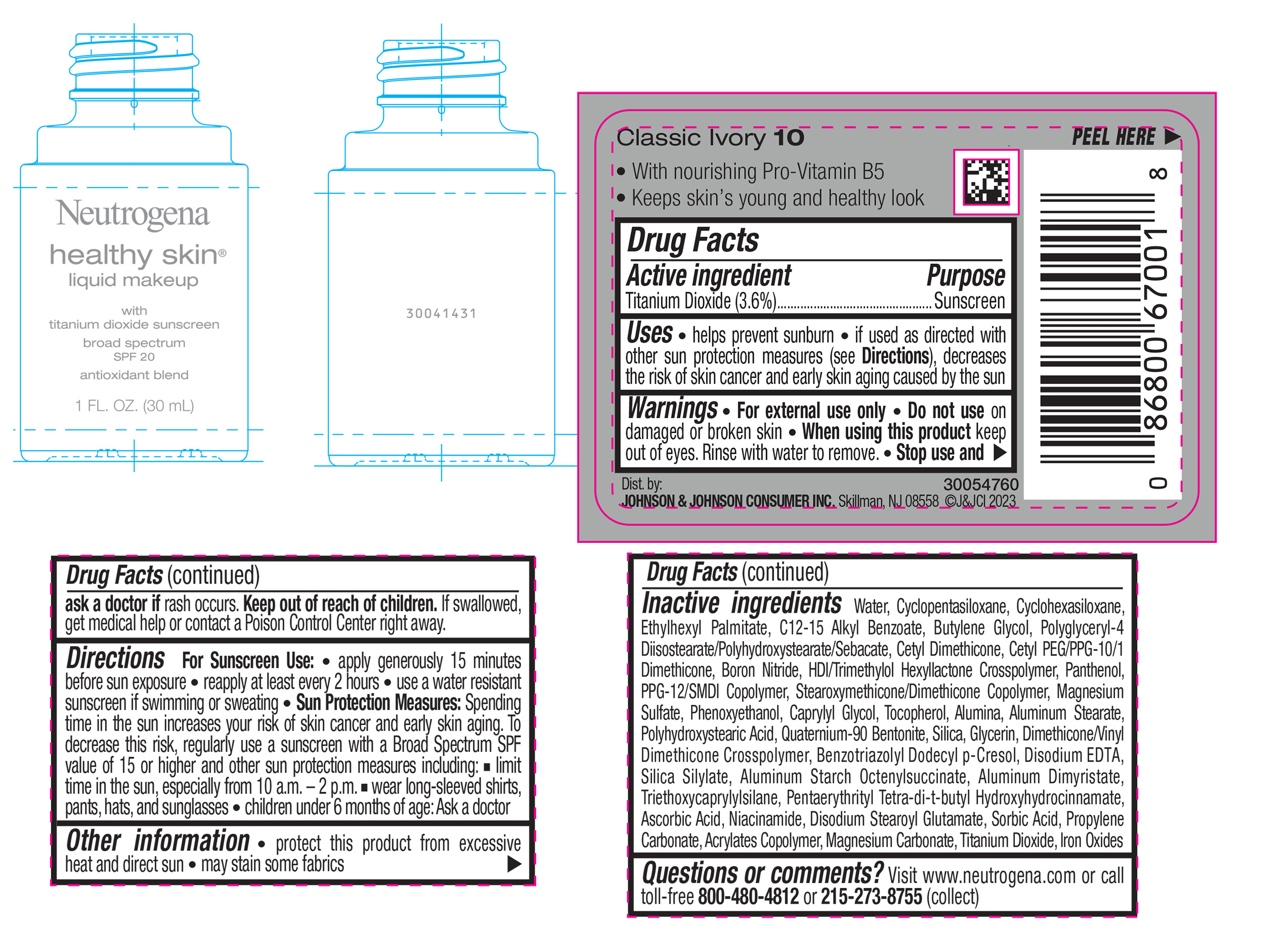
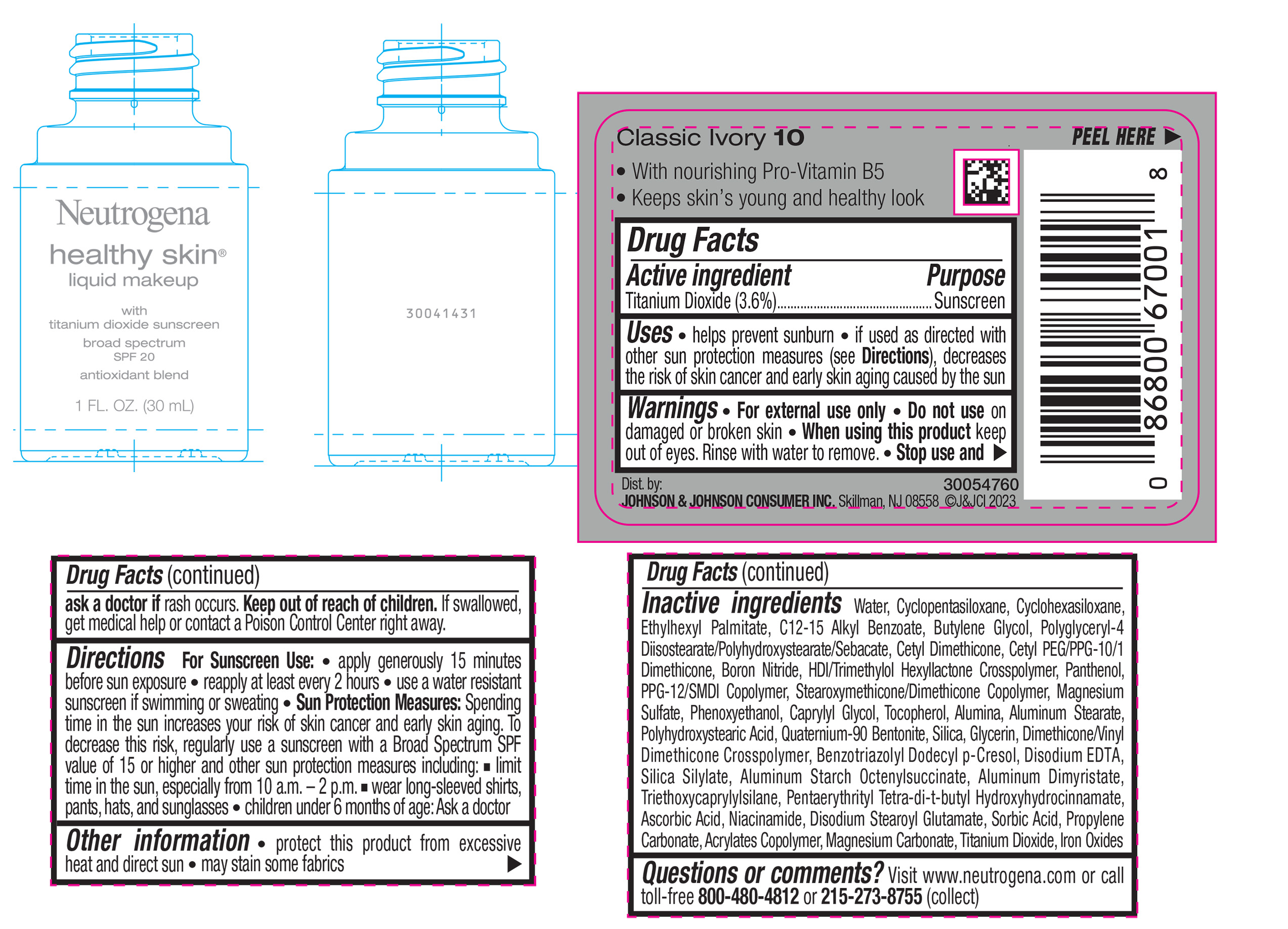
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CLASSIC IVORY 10- titanium dioxide liquid
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL IVORY 20- titanium dioxide liquid
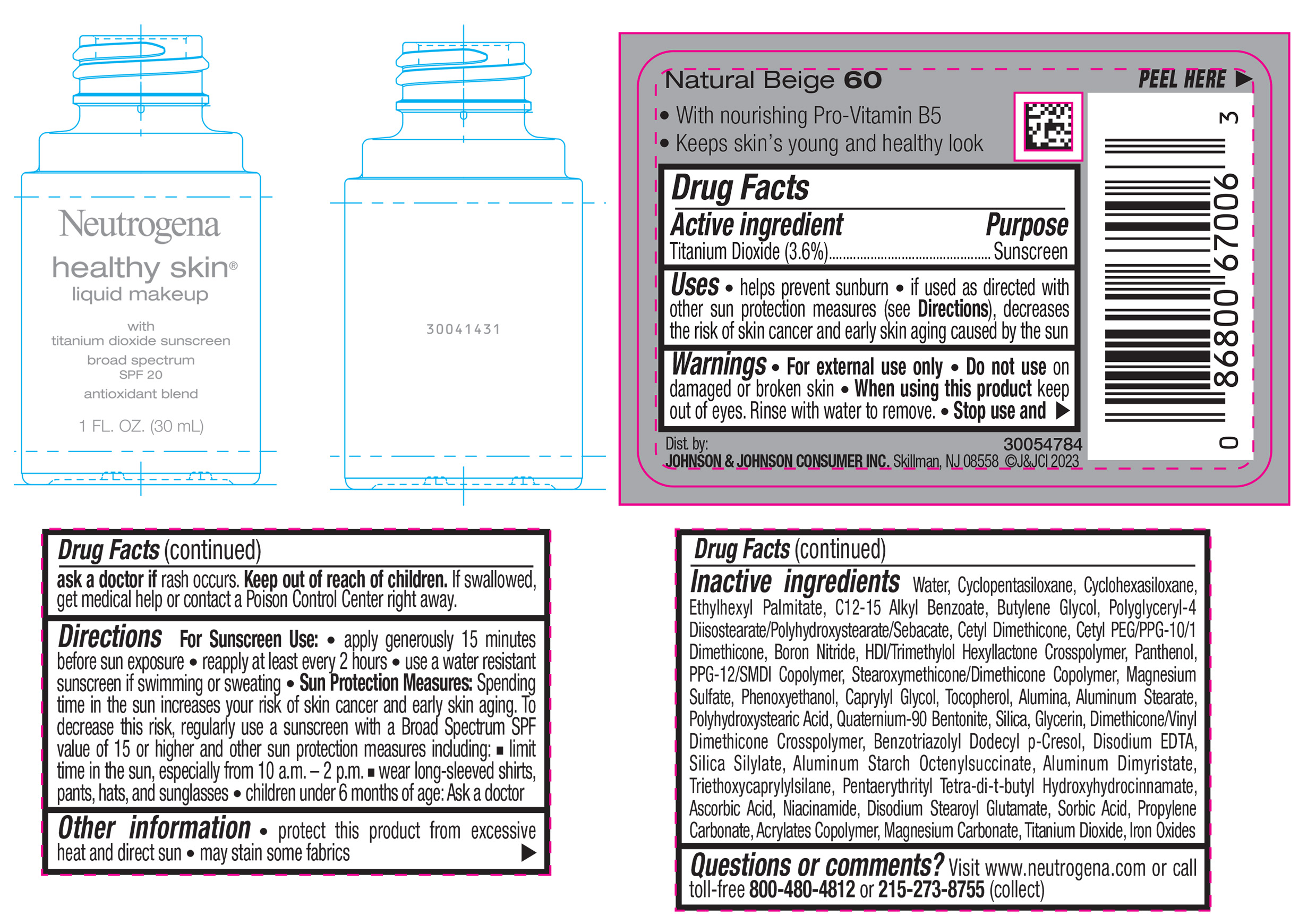
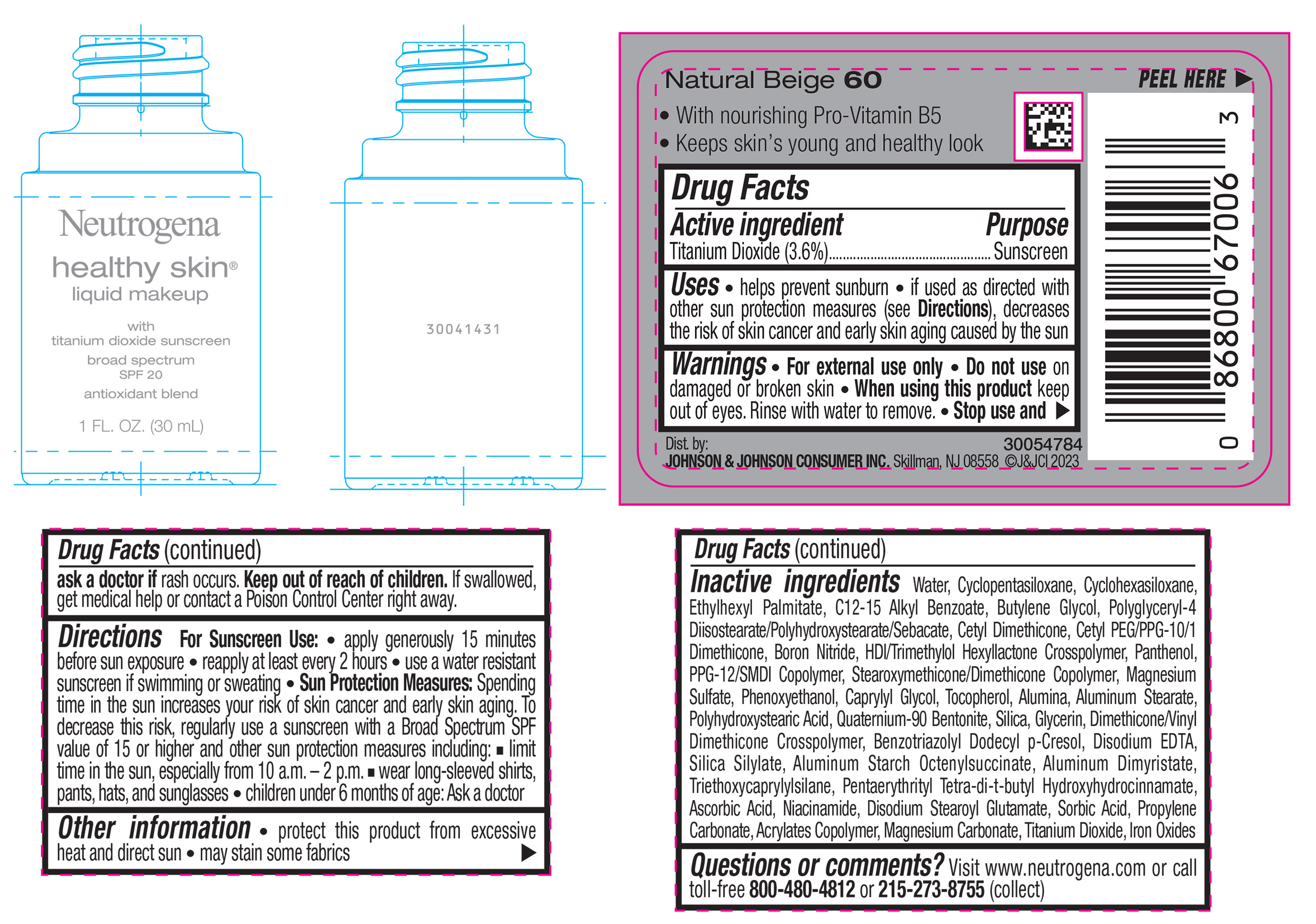
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL BEIGE 60- titanium dioxide liquid
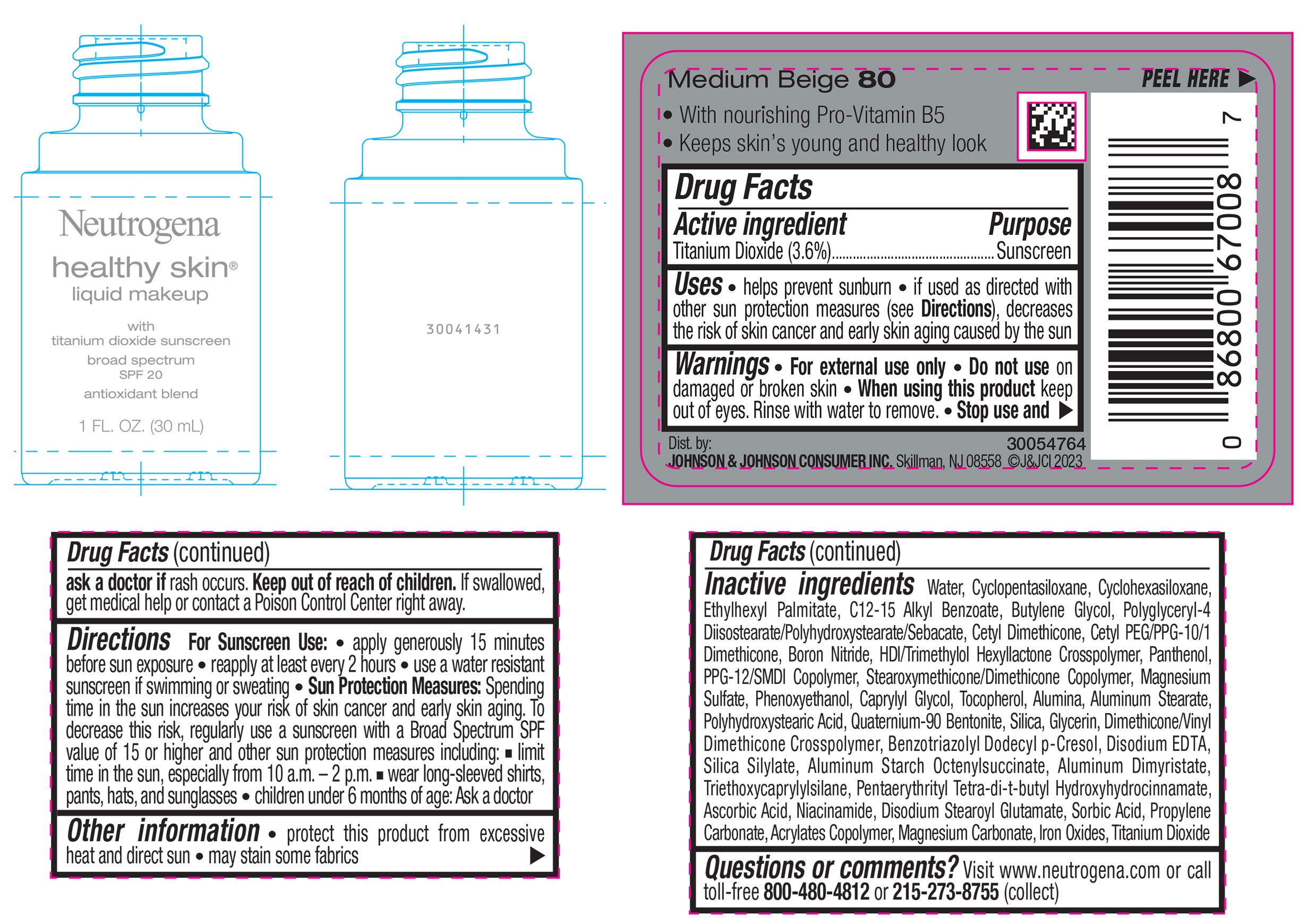
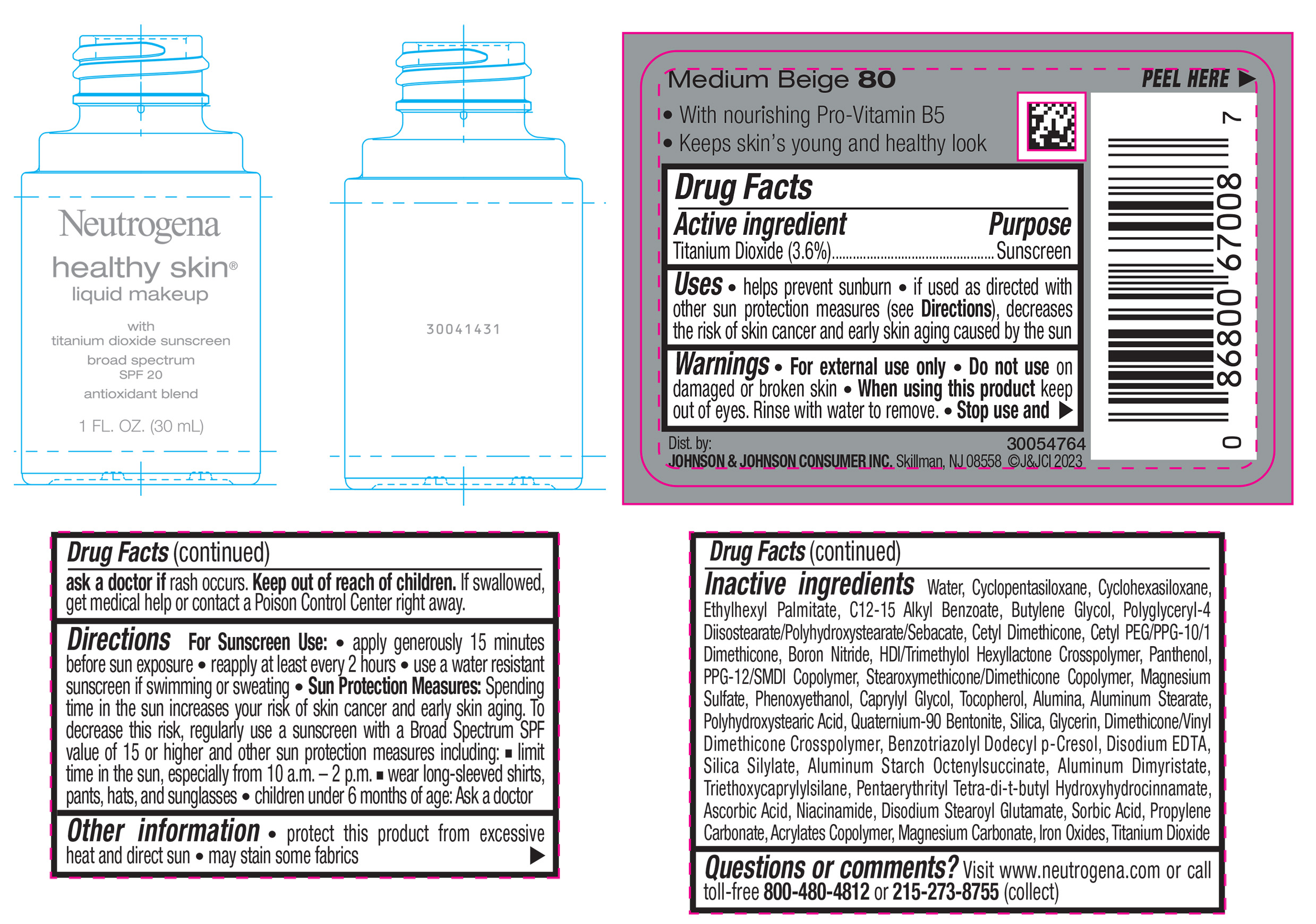
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - MEDIUM BEIGE 80- titanium dioxide liquid
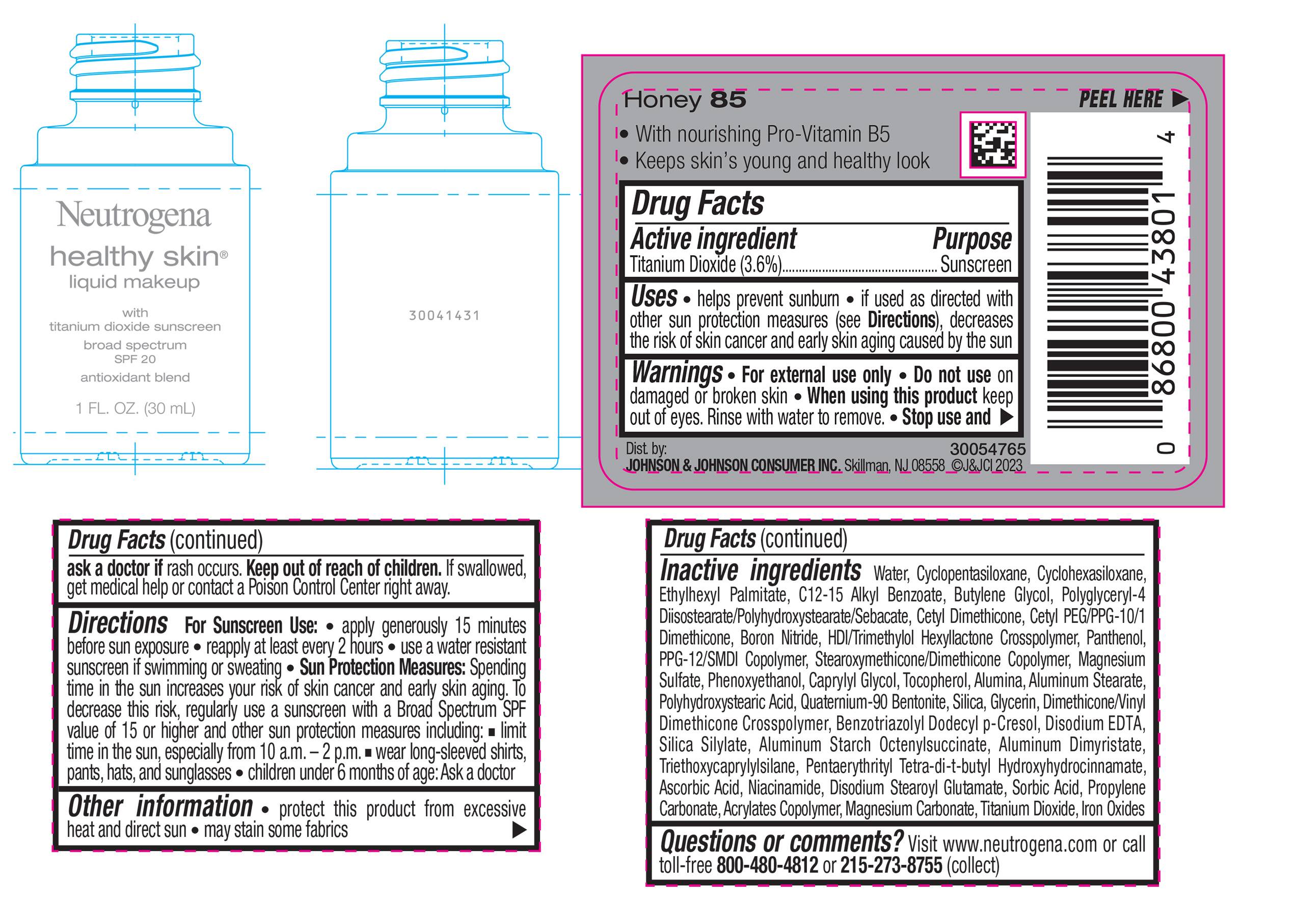
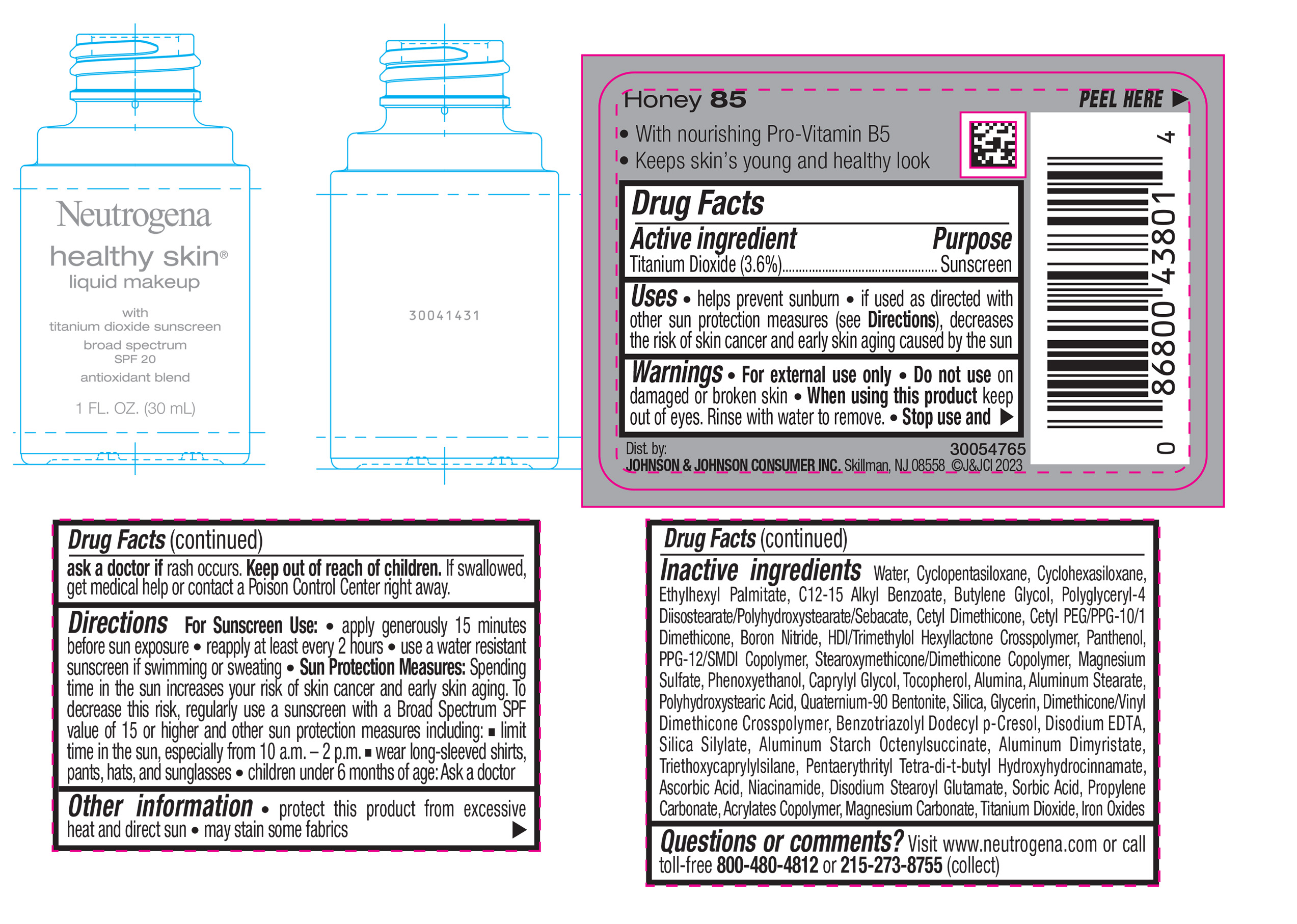
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - HONEY 85- titanium dioxide liquid
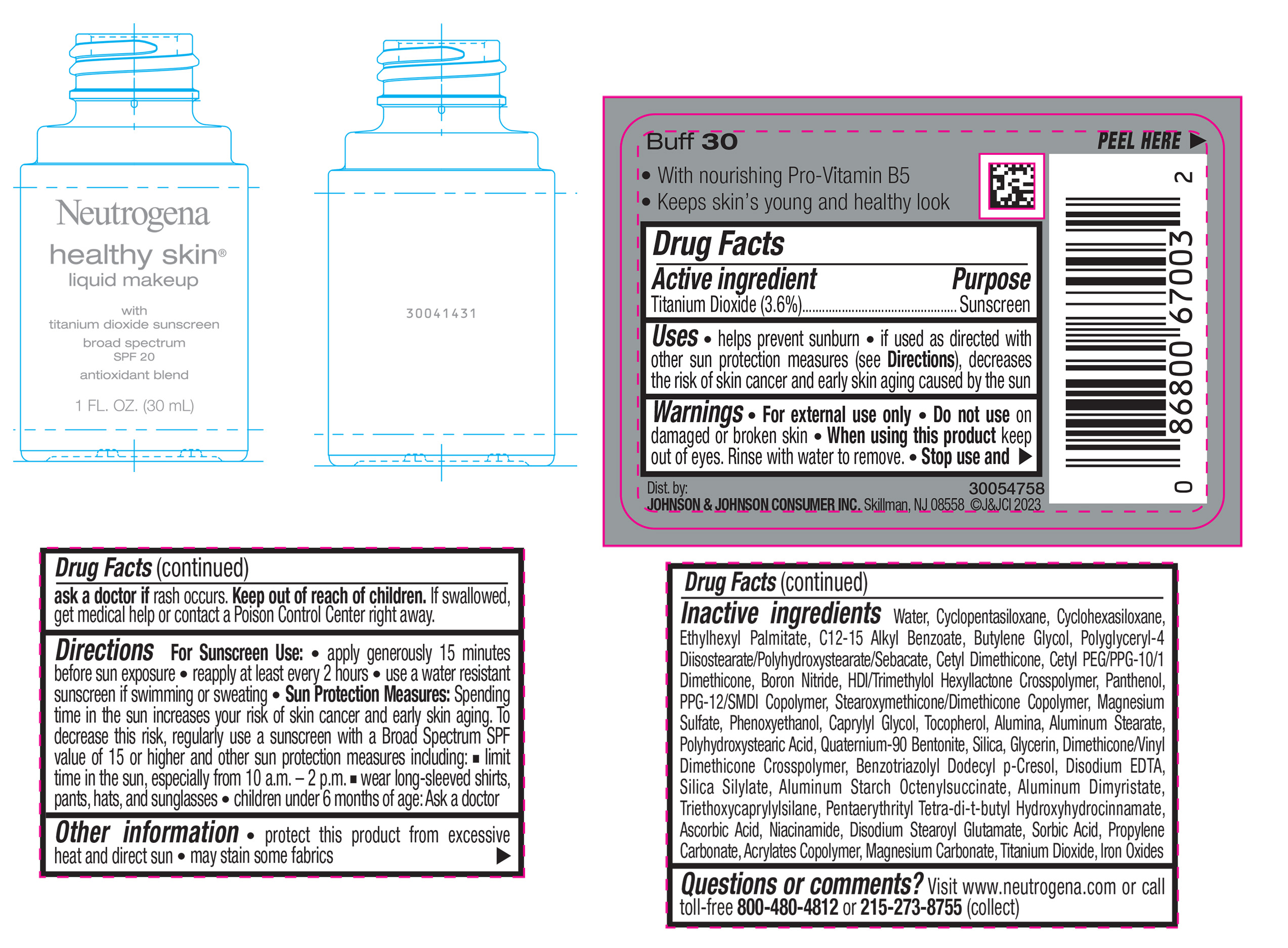
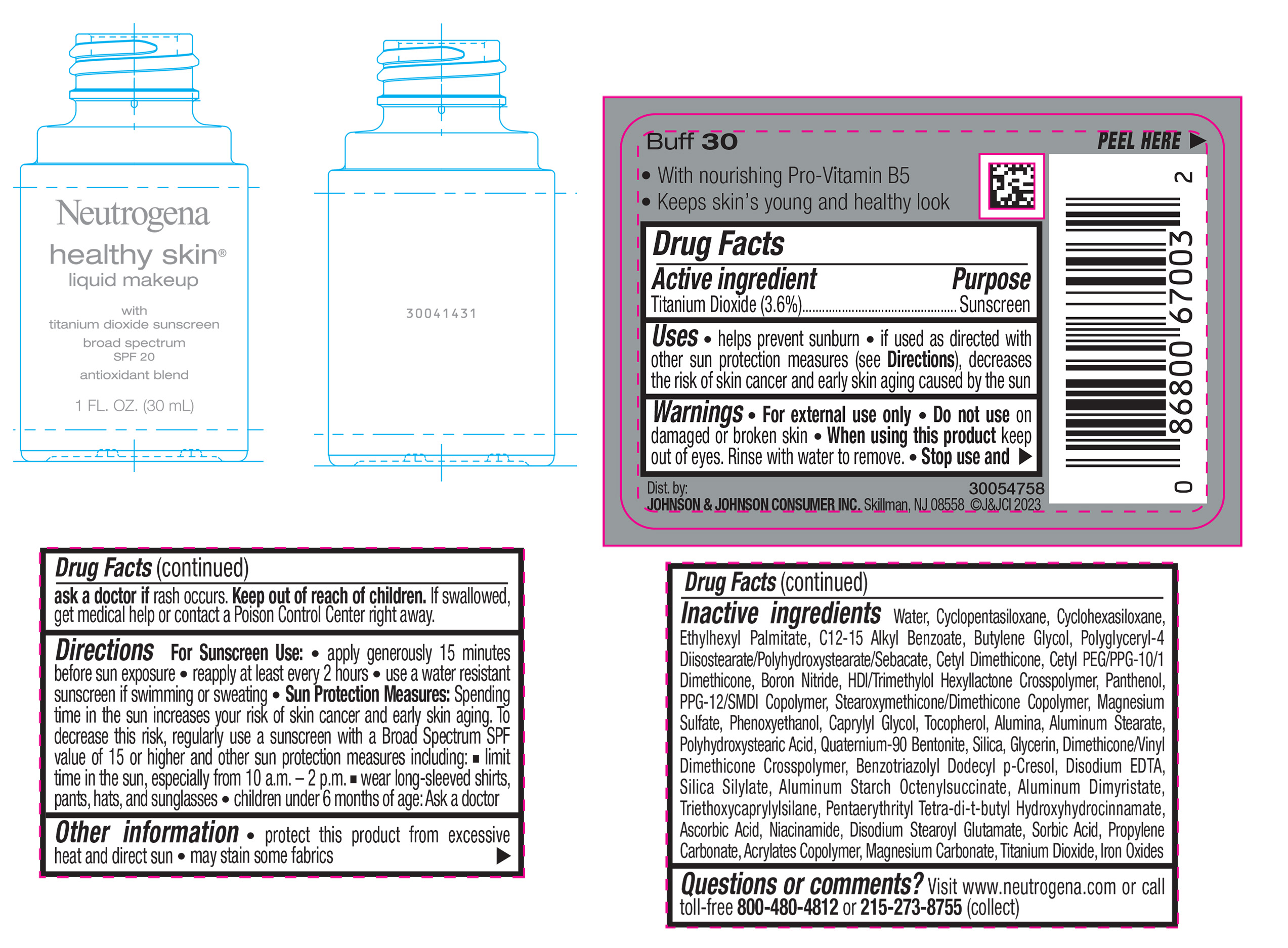
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - BUFF 30- titanium dioxide liquid
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NUDE 40- titanium dioxide liquid
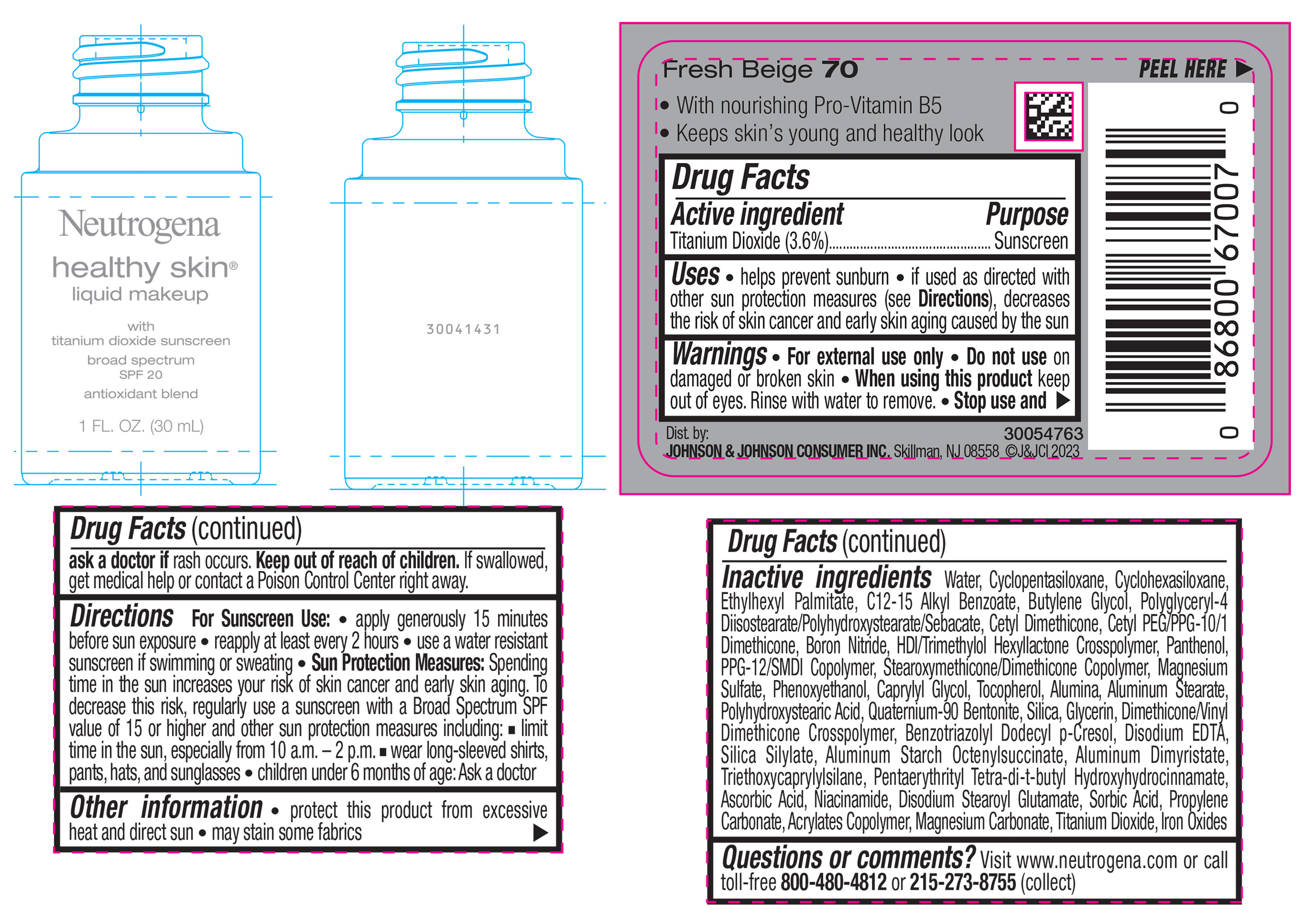
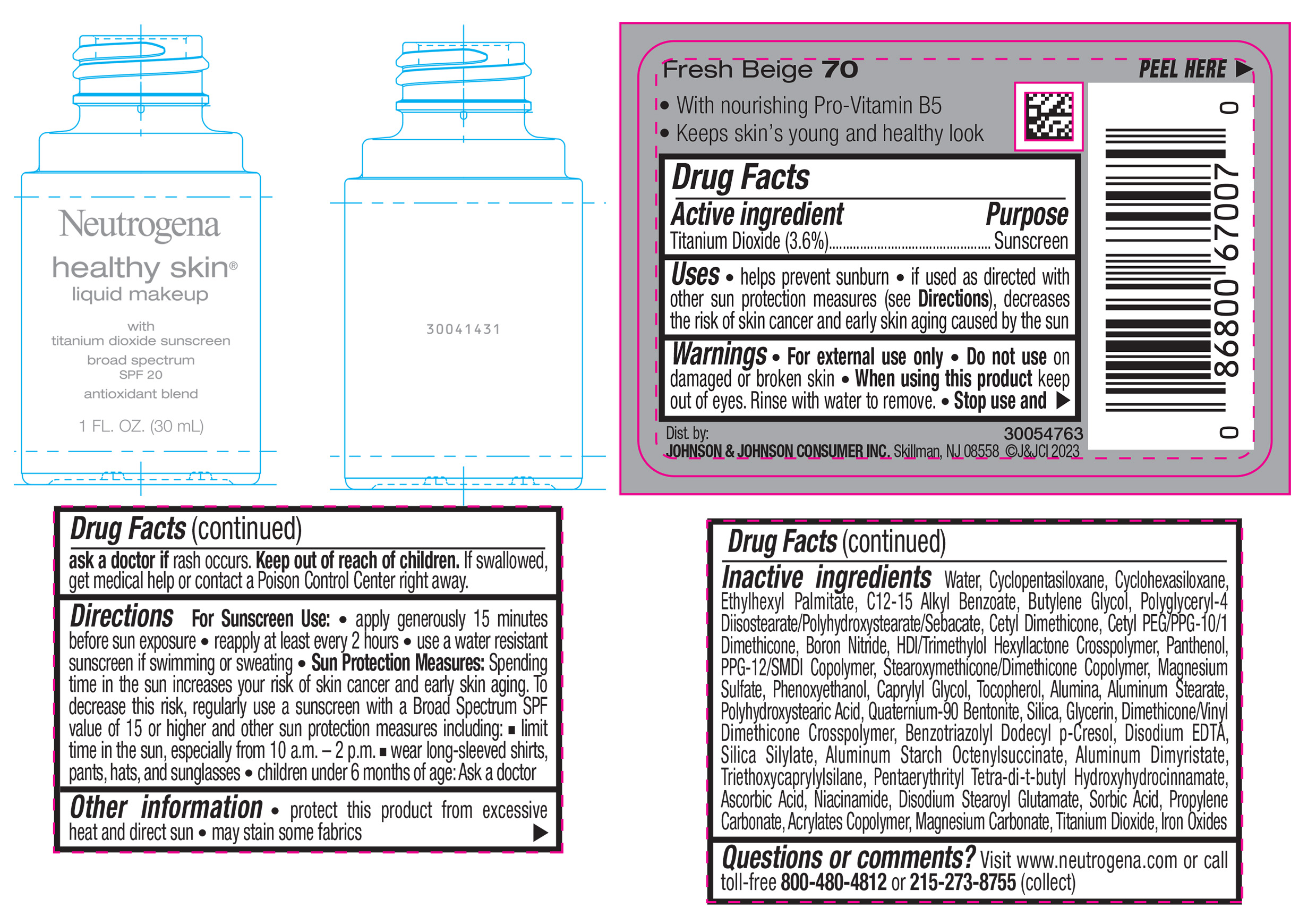
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - FRESH BEIGE 70- titanium dioxide liquid
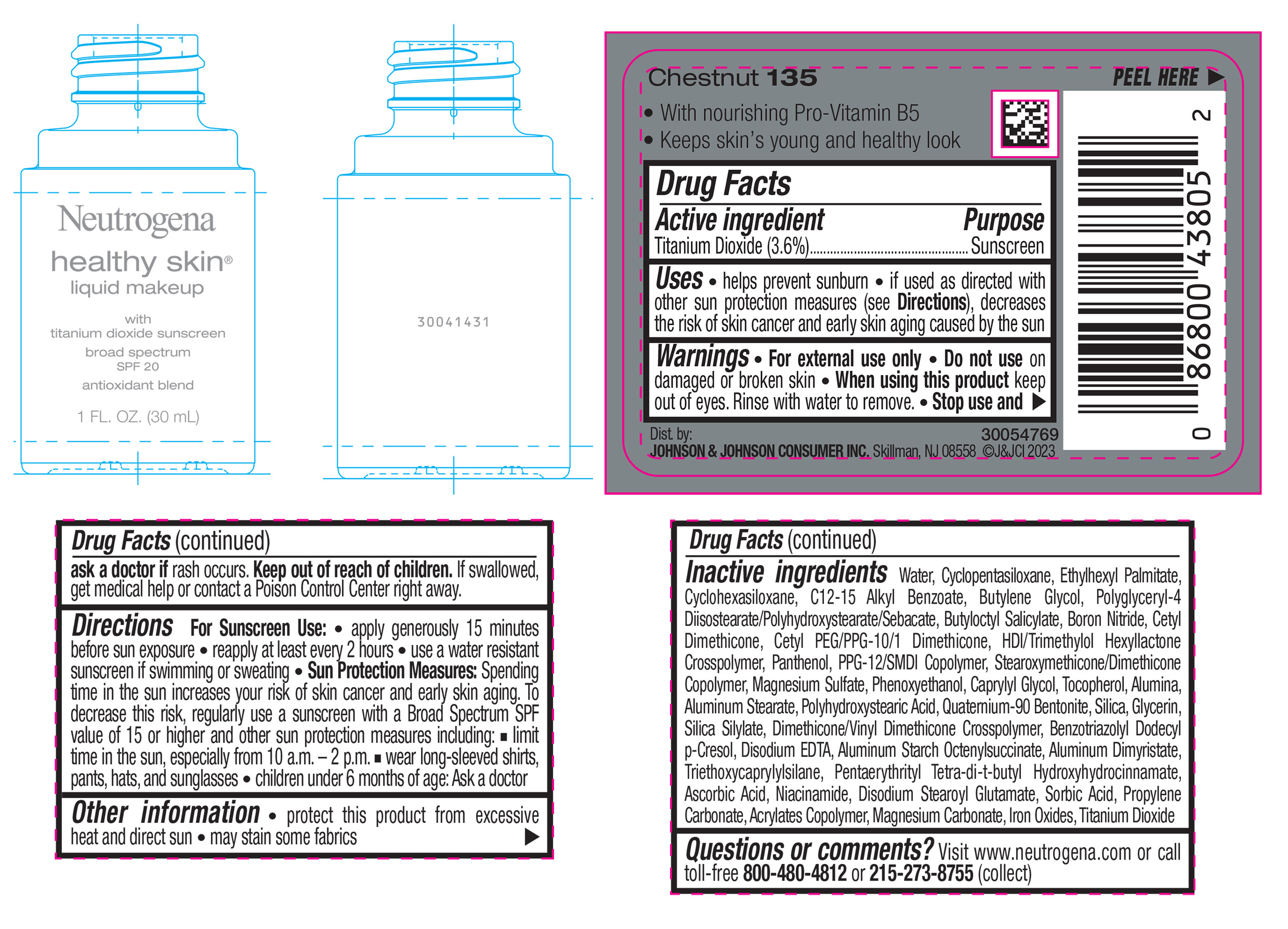
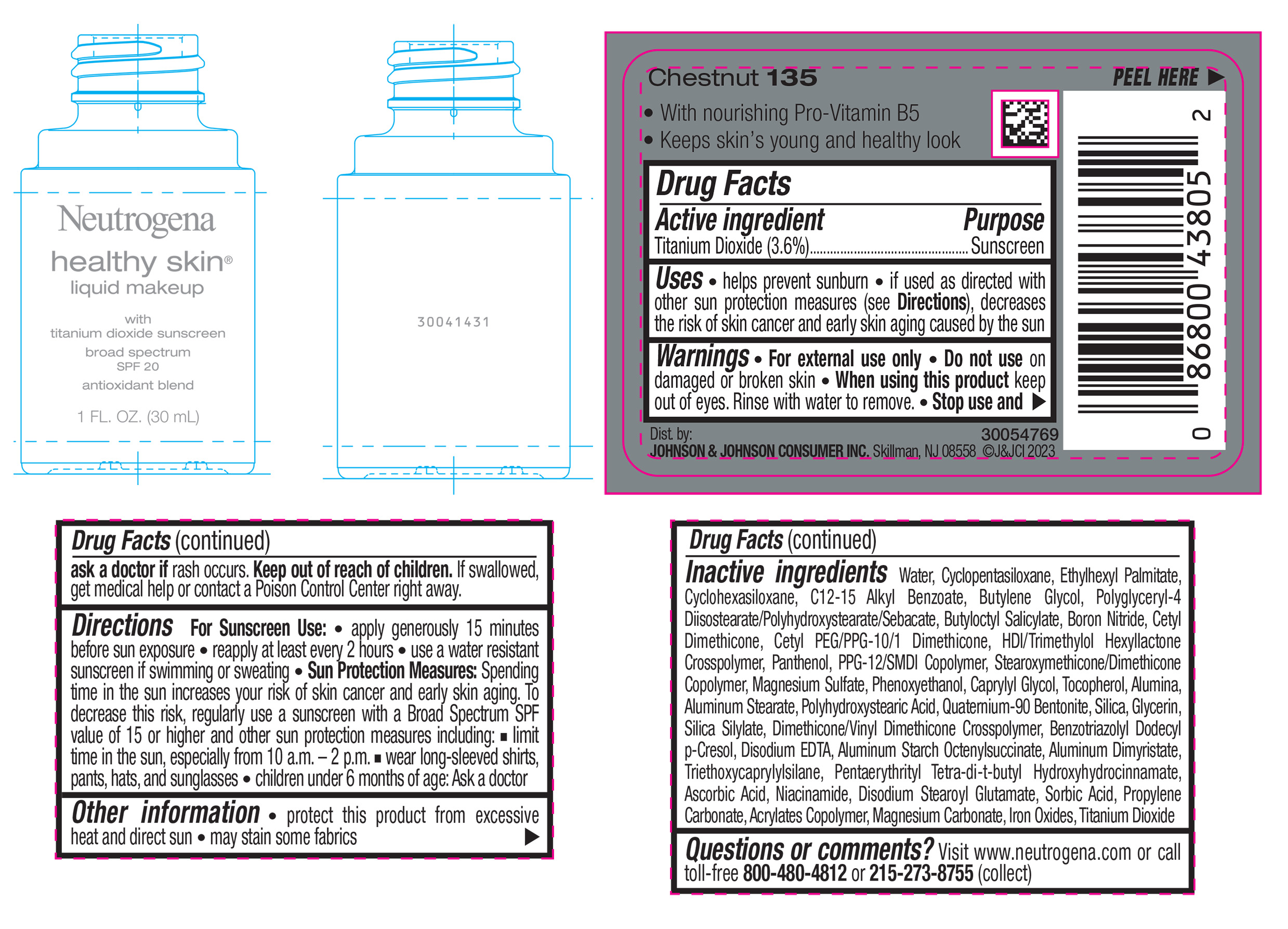
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CHESTNUT 135- titanium dioxide liquid
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - WARM BEIGE 90- titanium dioxide liquid
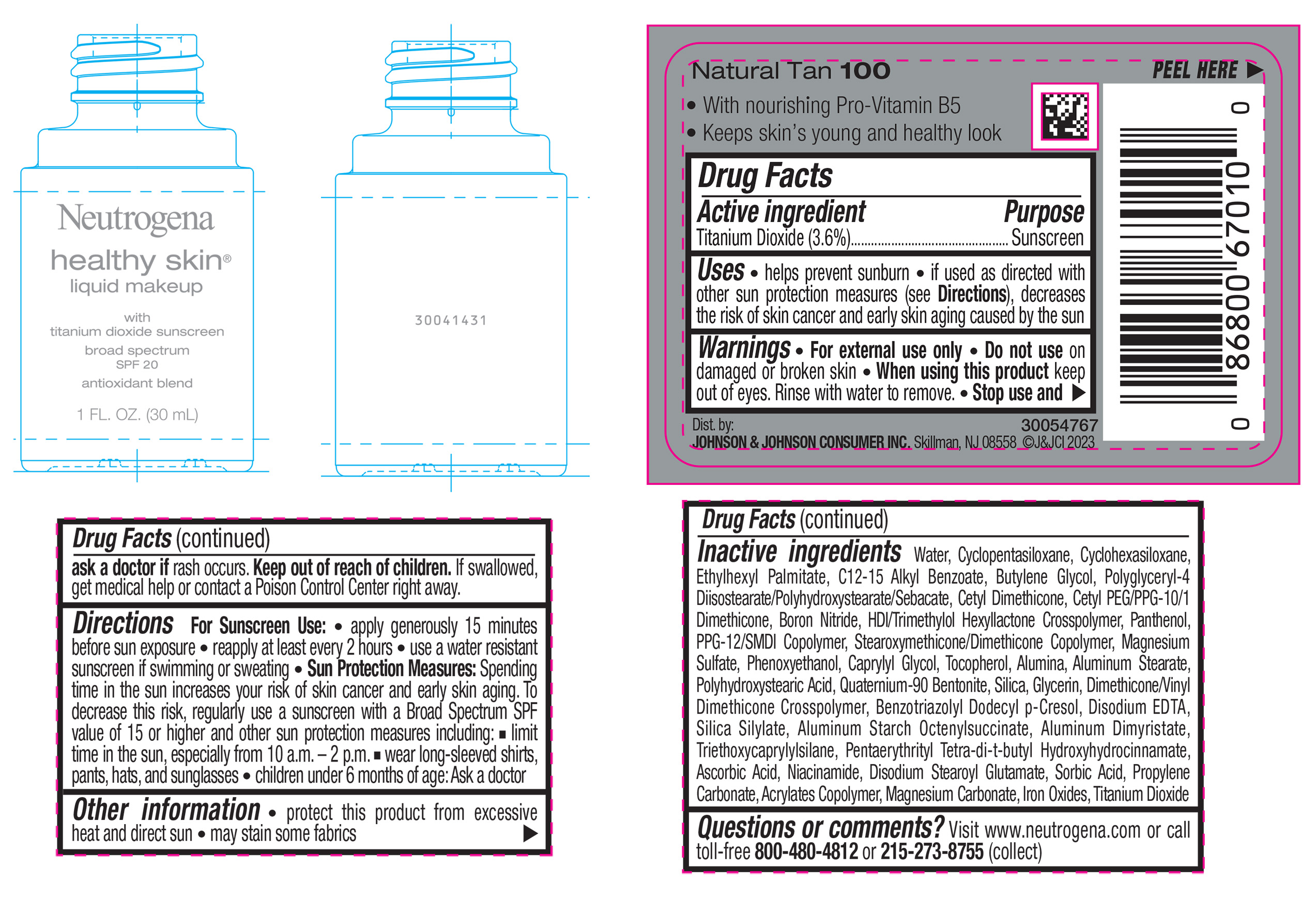
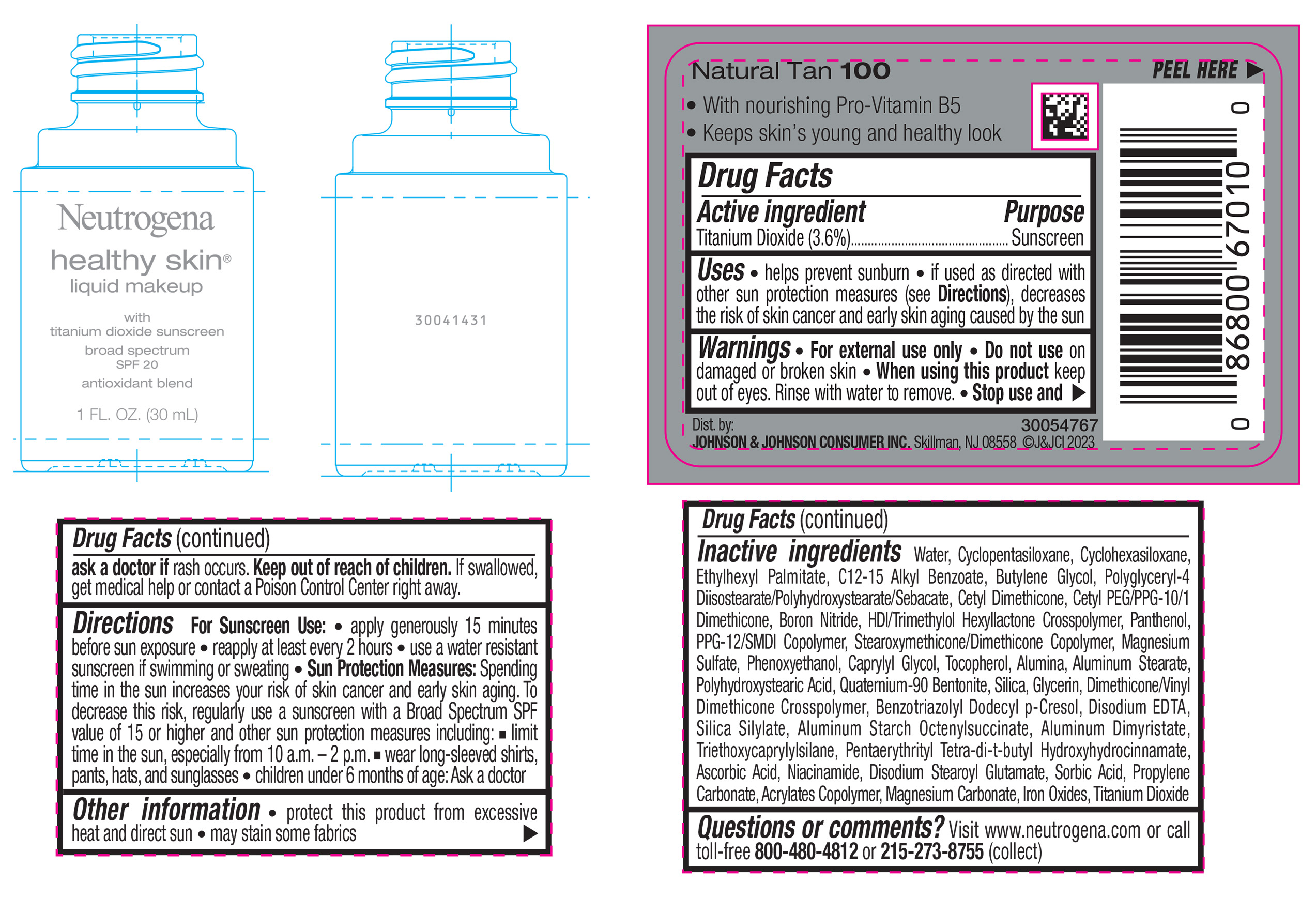
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL TAN 100- titanium dioxide liquid
-
NDC Code(s):
69968-0867-1,
69968-0868-1,
69968-0869-1,
69968-0870-1, view more69968-0871-1, 69968-0872-1, 69968-0873-1, 69968-0874-1, 69968-0875-1, 69968-0876-1, 69968-0877-1, 69968-0878-1, 69968-0879-1, 69968-0880-1
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
For Sunscreen Use:
• apply generously 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
▪ limit time in the sun, especially from 10 a.m. – 2 p.m.
▪ wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Water, Cyclopentasiloxane, Cyclohexasiloxane, Ethylhexyl Palmitate, C12-15 Alkyl Benzoate, Butylene Glycol, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Cetyl Dimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Boron Nitride, HDI/Trimethylol Hexyllactone Crosspolymer, Panthenol, PPG-12/SMDI Copolymer, Stearoxymethicone/Dimethicone Copolymer, Magnesium Sulfate, Phenoxyethanol, Caprylyl Glycol, Tocopherol, Alumina, Aluminum Stearate, Polyhydroxystearic Acid, Quaternium-90 Bentonite, Silica, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, Benzotriazolyl Dodecyl p-Cresol, Disodium EDTA, Silica Silylate, Aluminum Starch Octenylsuccinate, Aluminum Dimyristate, Triethoxycaprylylsilane, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Ascorbic Acid, Niacinamide, Disodium Stearoyl Glutamate, Sorbic Acid, Propylene Carbonate, Acrylates Copolymer, Magnesium Carbonate, Titanium Dioxide, Iron Oxides
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
For Sunscreen Use:
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
▪ limit time in the sun, especially from 10 a.m. – 2 p.m.
▪ wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Water, Cyclopentasiloxane, Cyclohexasiloxane, Ethylhexyl Palmitate, C12-15 Alkyl Benzoate, Butylene Glycol, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Cetyl Dimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Butyloctyl Salicylate, Boron Nitride, HDI/Trimethylol Hexyllactone Crosspolymer, Panthenol, PPG-12/SMDI Copolymer, Stearoxymethicone/ Dimethicone Copolymer, Magnesium Sulfate, Phenoxyethanol, Caprylyl Glycol, Tocopherol, Alumina, Aluminum Stearate, Polyhydroxystearic Acid, Quaternium-90 Bentonite, Silica, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, Benzotriazolyl Dodecyl p-Cresol, Disodium EDTA, Silica Silylate, Aluminum Starch Octenylsuccinate, Aluminum Dimyristate, Triethoxycaprylylsilane, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Ascorbic Acid, Niacinamide, Disodium Stearoyl Glutamate, Sorbic Acid, Propylene Carbonate, Acrylates Copolymer, Magnesium Carbonate, Titanium Dioxide, Iron Oxides
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Classic Ivory 10
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Ivory 20
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Buff 30
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Nude 40
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Soft Beige 50
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Beige 60
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Fresh Beige 70
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Medium Beige 80
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Warm Beige 90
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Natural Tan 100
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Honey 85
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Caramel 105
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Cocoa 115
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label - Chestnut 135
-
INGREDIENTS AND APPEARANCE
NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - SOFT BEIGE 50
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0871 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0871-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CARAMEL 105
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0878 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0878-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - COCOA 115
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0879 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0879-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CLASSIC IVORY 10
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0867 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0867-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL IVORY 20
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0868 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0868-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL BEIGE 60
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0872 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0872-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - MEDIUM BEIGE 80
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0874 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0874-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - HONEY 85
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0875 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0875-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - BUFF 30
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0869 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0869-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NUDE 40
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0870 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0870-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - FRESH BEIGE 70
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0873 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0873-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - CHESTNUT 135
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0880 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0880-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 05/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/20/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - WARM BEIGE 90
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0876 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0876-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 NEUTROGENA HEALTHY SKIN LIQUID MAKEUP BROAD SPECTRUM SPF 20 - NATURAL TAN 100
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0877 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL PALMITATE (UNII: 2865993309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLENE CARBONATE (UNII: 8D08K3S51E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) FERROUS OXIDE (UNII: G7036X8B5H) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) WATER (UNII: 059QF0KO0R) TOCOPHEROL (UNII: R0ZB2556P8) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BENZOTRIAZOLYL DODECYL P-CRESOL (UNII: 298PX4M11X) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) MAGNESIUM CARBONATE (UNII: 0E53J927NA) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) EDETATE DISODIUM (UNII: 7FLD91C86K) NIACINAMIDE (UNII: 25X51I8RD4) SORBIC ACID (UNII: X045WJ989B) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0877-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/29/2024 Labeler - Kenvue Brands LLC (118772437)