Label: NAIL PROTECT PATCHES- acrylates copolymer patch
- NDC Code(s): 84037-0071-1, 84037-0071-2
- Packager: Guangzhou Zhicunxiu Cosmetics Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
When using this product
keep away from eyes, lips and mouth. lf contact occurs, flush thoroughly with water.
skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time If irritation occurs, only use one topical acne medication at a time.
- Keep out of reach of children.
-
Directions
- cleanse skin thoroughly before applying this product
- apply patch(es) to affected area(s)
- to remove, gently peel back patch and discard
- because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
- Inactive Ingredients
- Questions?
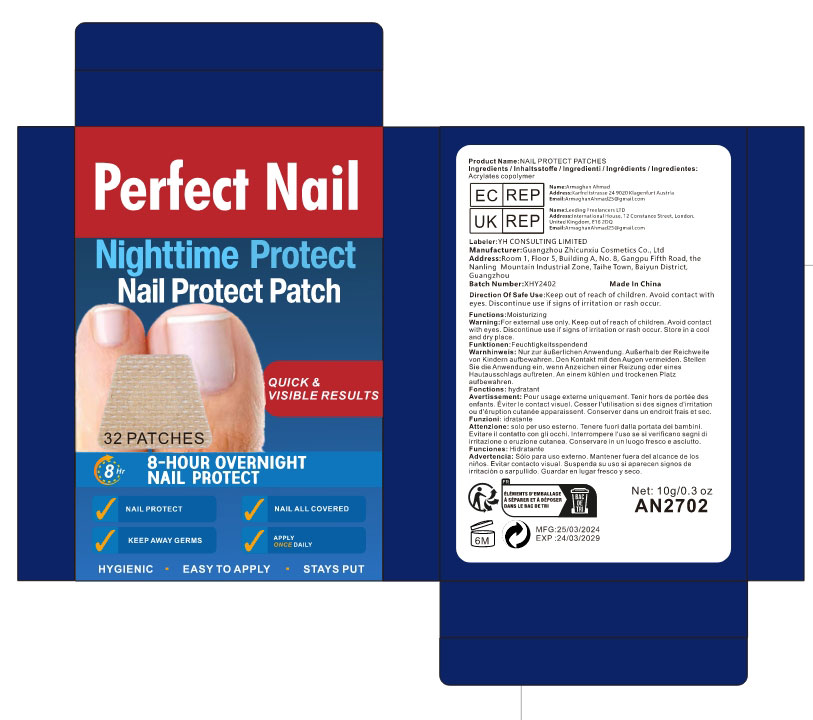
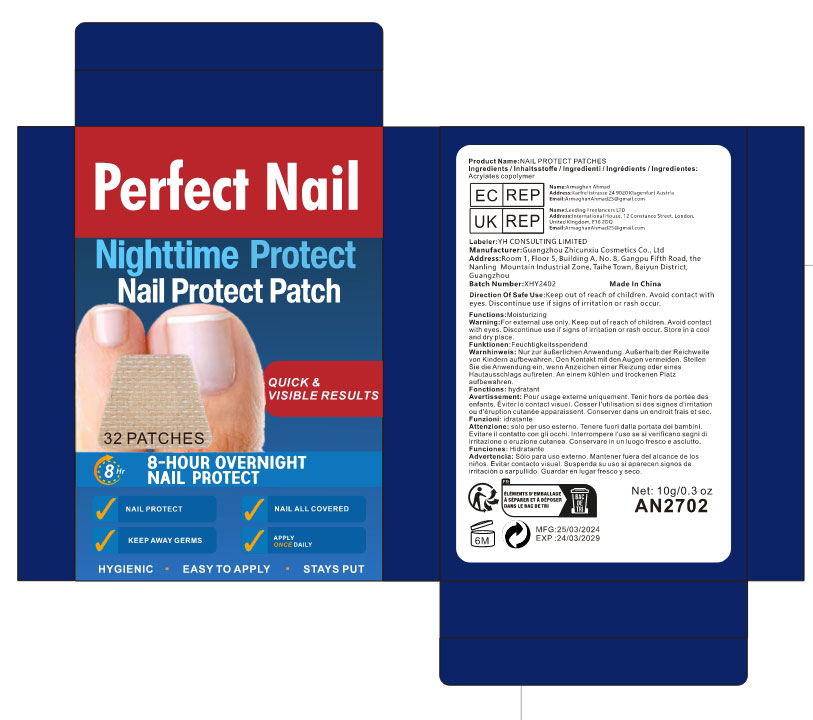
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAIL PROTECT PATCHES
acrylates copolymer patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84037-0071 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACRYLATES CROSSPOLYMER-6 (UNII: 4GXD0Q3OS3) (ACRYLATES CROSSPOLYMER-6 - UNII:4GXD0Q3OS3) ACRYLATES CROSSPOLYMER-6 5 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84037-0071-1 32 in 1 BOX; Type 0: Not a Combination Product 03/22/2024 2 NDC:84037-0071-2 72 in 1 BOX; Type 0: Not a Combination Product 03/21/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/21/2024 Labeler - Guangzhou Zhicunxiu Cosmetics Co., Ltd (228356123)