Label: ADAPALENE AND BENZOYL PEROXIDE gel
- NDC Code(s): 51672-1384-4, 51672-1384-9
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADAPALENE AND BENZOYL PEROXIDE TOPICAL GEL safely and effectively. See full prescribing information for ADAPALENE AND BENZOYL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAdapalene and benzoyl peroxide topical gel is indicated for the topical treatment of acne vulgaris in adults and pediatric patients 12 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Adapalene and benzoyl peroxide topical gel is not for oral, ophthalmic, or intravaginal use. Apply a thin layer of adapalene and benzoyl peroxide topical gel to affected ...
-
3 DOSAGE FORMS AND STRENGTHSEach gram of adapalene and benzoyl peroxide topical gel contains 3 mg (0.3%) adapalene and 25 mg (2.5%) benzoyl peroxide in a white to pale yellow, opaque gel. Adapalene and benzoyl peroxide ...
-
4 CONTRAINDICATIONSAdapalene and benzoyl peroxide topical gel is contraindicated in patients with a history of hypersensitivity reactions to benzoyl peroxide or any components of the formulation in adapalene and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria, have been reported with the use of benzoyl peroxide products. If a serious hypersensitivity ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - The following adverse reactions are discussed in greater detail elsewhere in the labeling: Hypersensitivity - [see - Warnings and Precautions (5.1) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available pharmacovigilance data with adapalene and benzoyl peroxide topical gel use in pregnant women are insufficient to establish a drug-associated risk of ...
-
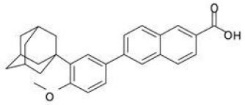
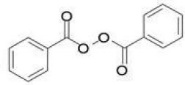
11 DESCRIPTIONAdapalene and benzoyl peroxide topical gel, 0.3%/2.5% is a white to pale yellow, opaque gel for topical use containing adapalene 0.3% and benzoyl peroxide 2.5%. Adapalene, a synthetic retinoid, is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Adapalene - Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, genotoxicity, or fertility studies were conducted with adapalene and benzoyl peroxide topical gel. Carcinogenicity ...
-
14 CLINICAL STUDIESThe safety and efficacy of adapalene and benzoyl peroxide topical gel, 0.3%/2.5% applied once daily for 12 weeks for the treatment of acne vulgaris were assessed in a multicenter, randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Adapalene and benzoyl peroxide topical gel 0.3% / 2.5% is white to pale yellow in color and opaque in appearance, and is supplied as follows: 45 gram pump - NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA approved patient labeling (Patient Information). Hypersensitivity - Inform patients that serious hypersensitivity reactions occurred with the use of benzoyl ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc.,Hawthorne, NY 10532 - Revised: December 2022 - 5235292 21 ...
-
PATIENT PACKAGE INSERTPatient Information - Adapalene (a dap' a leen) and Benzoyl Peroxide - (BEN-zoe-il per-OX-ide) Topical Gel - This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 45 g Bottle CartonNDC 51672-1384-9 - 45 g - Adapalene and - Benzoyl Peroxide - Topical Gel 0.3% / 2.5% PUMP - FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE. Rx only - Keep this and all ...
-
INGREDIENTS AND APPEARANCEProduct Information