Label: TIROFIBAN HYDROCHLORIDE injection, solution
- NDC Code(s): 25021-417-84
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIROFIBAN HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for TIROFIBAN HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Tirofiban hydrochloride injection is indicated to reduce the rate of thrombotic cardiovascular events (combined endpoint of death, myocardial infarction, or refractory ischemia/repeat cardiac ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - The recommended dosage is 25 mcg/kg administered intravenously within 5 minutes and then 0.15 mcg/kg/min for up to 18 hours. 2.2 Administration - For intravenous use ...
-
3 DOSAGE FORMS AND STRENGTHS
Tirofiban hydrochloride injection 12.5 mg tirofiban per 250 mL (50 mcg per mL) is a clear, non-preserved, colorless, isosmotic, sterile premixed injection with sodium chloride for tonicity ...
-
4 CONTRAINDICATIONS
Tirofiban hydrochloride injection is contraindicated in patients with: Severe hypersensitivity reaction to tirofiban hydrochloride injection (i.e., anaphylactic reactions) [see Adverse Reactions ...
-
5 WARNINGS AND PRECAUTIONS
5.1 General Risk of Bleeding - Bleeding is the most common complication encountered during therapy with tirofiban hydrochloride injection. Most bleeding associated with tirofiban hydrochloride ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
Concomitant use of fibrinolytics, anticoagulants and antiplatelet drugs increases the risk of bleeding.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - While published data cannot definitively establish the absence of risk, available published case reports have not established an association with tirofiban use ...
-
10 OVERDOSAGE
In clinical trials, inadvertent overdosage with tirofiban hydrochloride injection occurred in doses up to 2 times the recommended dose for initial infusion doses. Inadvertent overdosage occurred ...
-
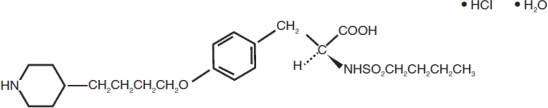
11 DESCRIPTION
Tirofiban hydrochloride injection contains tirofiban hydrochloride, a non-peptide antagonist of the platelet GP IIb/IIIa receptor, which inhibits platelet aggregation. Tirofiban hydrochloride ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Tirofiban hydrochloride injection is a reversible antagonist of fibrinogen binding to the GP IIb/IIIa receptor, the major platelet surface receptor involved in ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The carcinogenic potential of tirofiban hydrochloride injection has not been evaluated. Tirofiban HCl was negative in the in vitro ...
-
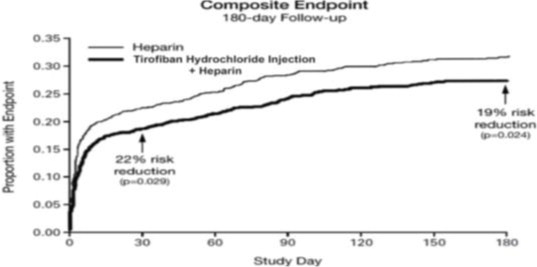
14 CLINICAL STUDIES
Two large-scale clinical studies established the efficacy of tirofiban hydrochloride injection in the treatment of patients with NSTE-ACS (unstable angina/non-ST elevation MI). The two studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tirofiban hydrochloride injection is a clear, non-preserved, colorless, isosmotic, sterile premixed solution with sodium chloride for tonicity adjustment and is supplied as ...
-
17 PATIENT COUNSELING INFORMATION
Advise patients to watch closely for any signs of bleeding or bruising and to report these to their health care provider when they occur. Advise patients to discuss with their health care ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Bag Label - NDC 25021-417-84 - 250 mL - Single-Dose Container - Tirofiban Hydrochloride Injection - 12.5 mg per 250 mL - (50 mcg per mL) Rx only - RECOMMENDED ...
-
INGREDIENTS AND APPEARANCEProduct Information