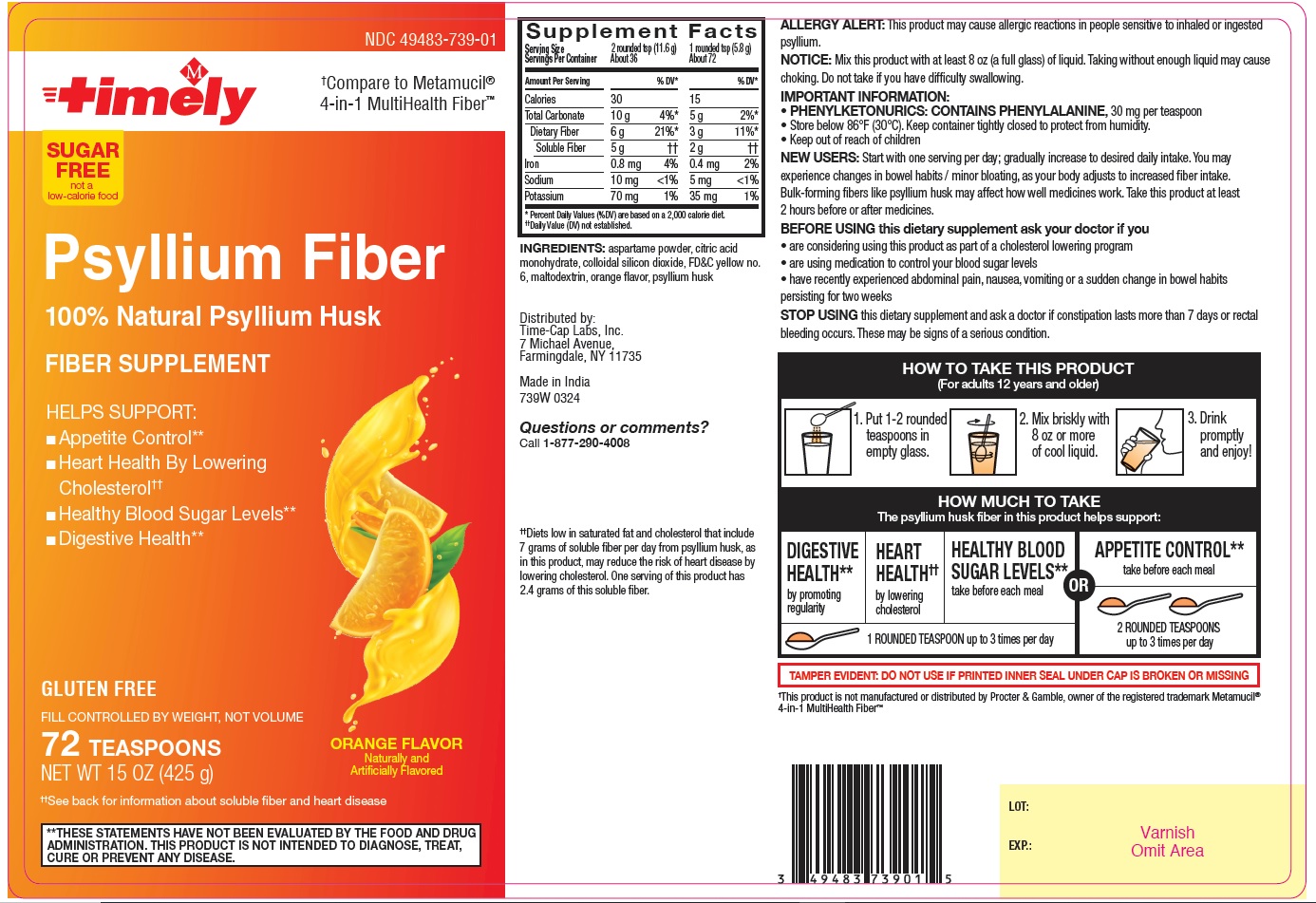
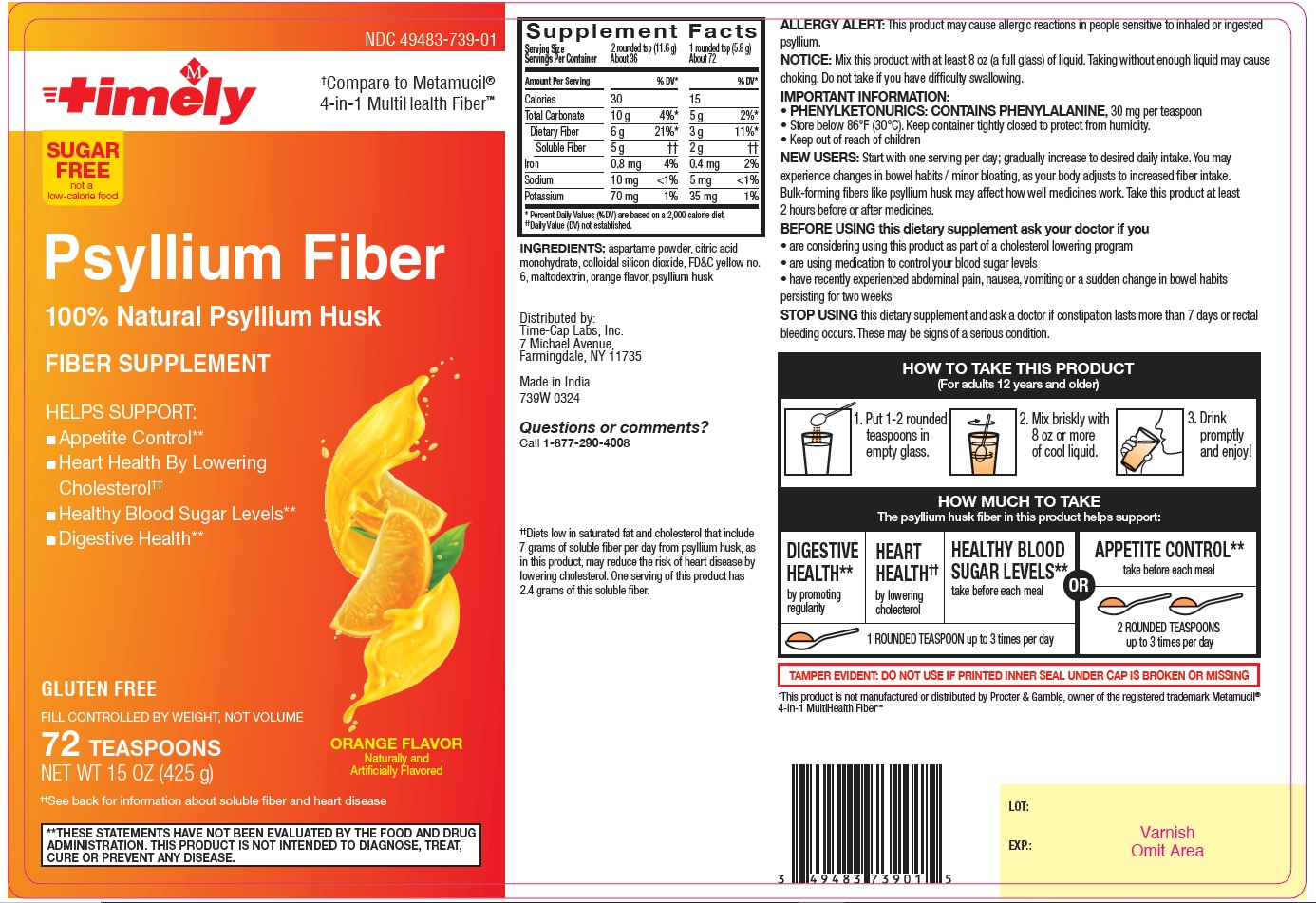
Label: PSYLLIUM FIBER- psyllium husk powder
- NDC Code(s): 49483-739-01
- Packager: TIME CAP LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Supplement Facts
Serving Size 2 rounded tsp (11.6 g) 1 rounded tsp (5.8 g) Servings Per Container About 36 About 72 Amount per Serving % DV* % DV* Calories 30 15 Total Carbonate 10g 4%* 5 g 2%* Dietary Fiber 6 g 21%* 3 g 11%* Soluble Fiber 5 g †† 2 g †† Iron 0.8 mg 4% 0.4 mg 2% Sodium 10 mg <1% 5 mg <1% Potassium 70 mg 1% 35 mg 1% *Percent Daily Values (%DV) are based on a 2,000 calorie diet.
†† Daily Value (DV) not established.
- INACTIVE INGREDIENT
-
WARNINGS
ALLERGY ALERT: This product may cause allergic reactions in people sensitive to inhaled or ingested psyllium.
NOTICE: Mix this product with at least 8 oz (a full glass) of liquid. Taking without enough liquid may cause choking. Do not take if you have difficulty swallowing.
IMPORTANT INFORMATION:
PHENYLKETONURICS: CONTAINS PHENYLALANINE, 30 mg per teaspoon
Store below 86°F (30° C). Keep container tightly closed to protect from humidity.
Keep out of reach of childrenNEW USERS: Start with one serving per day; gradually increase to desired daily intake. You may experience changes in bowel habits/ minor bloating, as your body adjusts to increased fiber intake. Bulk-forming fibers like psyllium husk may affect how well medicines work. Take this product at least 2 hours before or after medicines.
BEFORE USINGthis dietary supplement ask your doctor if you
are considering using this product as part of a cholesterol lowering program
are using medication to control your blood sugar levels
have recently experienced abdominal pain, nausea, vomiting or a sudden change in bowel habits persisting for two weeksSTOP USING this dietary supplement and ask a doctor if constipation lasts more than 7 days or rectal bleeding occurs. These may be signs of a serious condition.
-
DOSAGE & ADMINISTRATION
HOW TO TAKE THIS PRODUCT
(For adults 12 years and older)
1. Put 1-2 rounded teaspoons in empty glass.
2. Mix briskly with 8 oz or more of cool liquid.
3. Drink promptly and enjoy!
HOW MUCH TO TAKE
The psyllium husk fiber in this product helps support:
DIGESTIVE HEALTH** by promoting regularity
HEART HEALTH†† by lowering cholesterol
HEALTHY BLOOD SUGAR LEVELS** take before each meal
1 ROUNDED TEASPOONS up to 3 times per day
OR
APPETITE CONTROL** take before each meal
2 ROUNDED TEASPOONS up to 3 times per day
** THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
††Diets low in saturated fat and cholesterol that include 7 grams of soluble fiber per day from psyllium husk, as in this product, may reduce the risk of heart disease by lowering cholesterol. One serving of this product has 2.4 grams of this soluble fiber.
- QUESTIONS
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PSYLLIUM FIBER
psyllium husk powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49483-739 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSYLLIUM HUSK (UNII: 0SHO53407G) (PSYLLIUM HUSK - UNII:0SHO53407G) PSYLLIUM HUSK 3.4 g in 5.8 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ASPARTAME (UNII: Z0H242BBR1) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color white (White to light brown) Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-739-01 425 g in 1 BOTTLE; Type 0: Not a Combination Product 03/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 03/11/2024 Labeler - TIME CAP LABORATORIES, INC. (037052099) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 677604129 manufacture(49483-739)