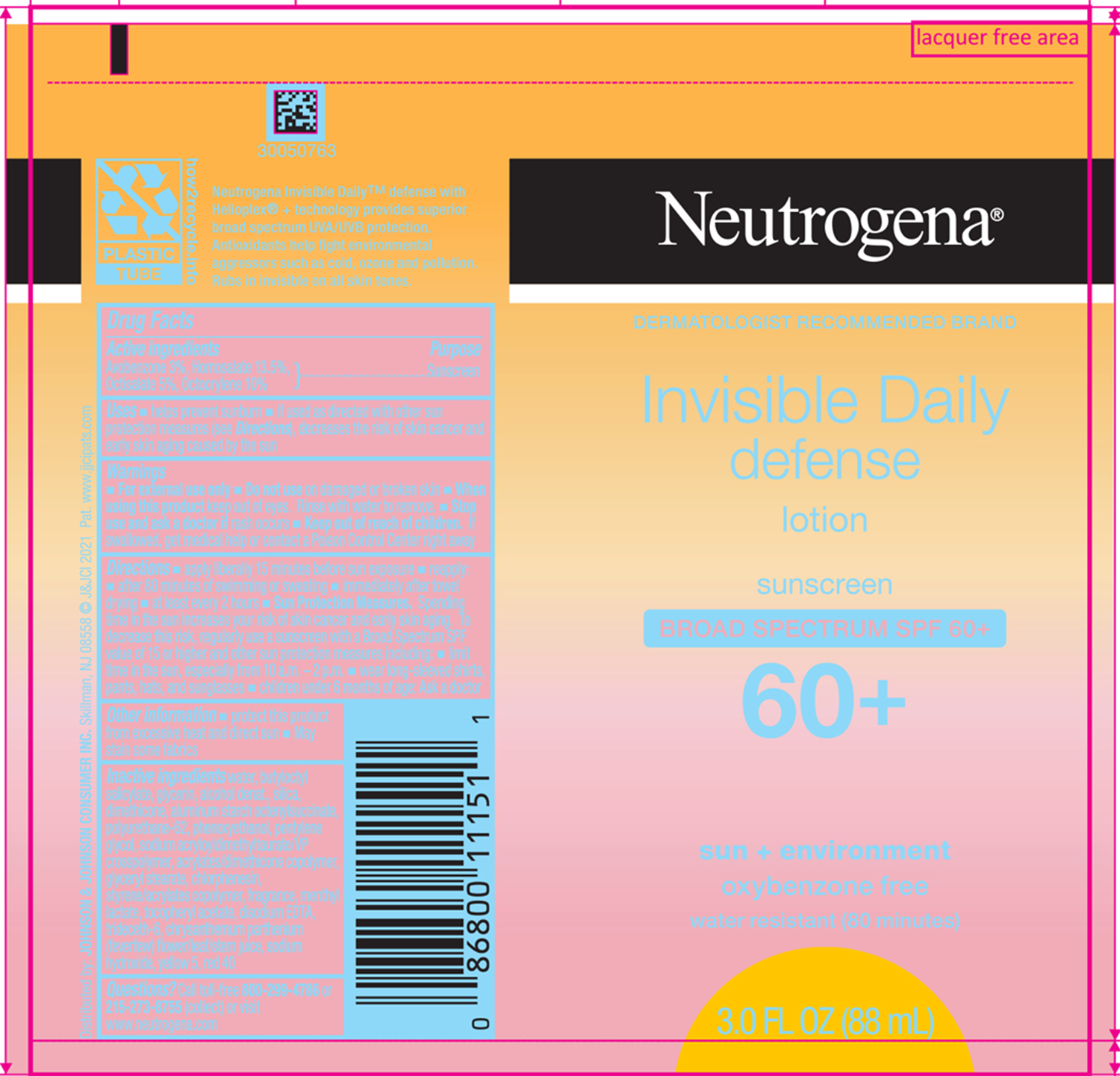
Label: NEUTROGENA INVISIBLE DAILY DEFENSE SUNSCREEN BROAD SPECTRUM SPF 60 PLUS- avobenzone, homosalate, octisalate, and octocrylene lotion
- NDC Code(s): 69968-0661-2, 69968-0661-3
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, butyloctyl salicylate, glycerin, alcohol denat., silica, dimethicone, aluminum starch octenylsuccinate, polyurethane-62, phenoxyethanol, pentylene glycol, sodium acryloyldimethyltaurate/VP crosspolymer, acrylates/dimethicone copolymer, glyceryl stearate, chlorphenesin, styrene/acrylates copolymer, fragrance, menthyl lactate, tocopheryl acetate, disodium EDTA, trideceth-6, chrysanthemum parthenium (feverfew) flower/leaf/stem juice, sodium hydroxide, yellow 5, red 40
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 88 mL Tube Label
-
INGREDIENTS AND APPEARANCE
NEUTROGENA INVISIBLE DAILY DEFENSE SUNSCREEN BROAD SPECTRUM SPF 60 PLUS
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0661 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 135 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE (UNII: 92RU3N3Y1O) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) PHENOXYETHANOL (UNII: HIE492ZZ3T) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CHLORPHENESIN (UNII: I670DAL4SZ) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) TRIDECETH-6 (UNII: 3T5PCR2H0C) FEVERFEW (UNII: Z64FK7P217) SODIUM HYDROXIDE (UNII: 55X04QC32I) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) POLYURETHANE-62 (UNII: TBK645J3J8) 2-ETHYLHEXYL ACRYLATE, METHACRYLATE, METHYL METHACRYLATE, OR BUTYL METHACRYLATE/HYDROXYPROPYL DIMETHICONE COPOLYMER (30000-300000 MW) (UNII: S7ZA3CCJ4M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0661-3 88 mL in 1 TUBE; Type 0: Not a Combination Product 10/05/2020 2 NDC:69968-0661-2 2 in 1 CARTON 10/05/2020 2 88 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/05/2020 Labeler - Kenvue Brands LLC (118772437)