Label: RANITIDINE tablet
- NDC Code(s): 55111-131-04, 55111-131-14, 55111-131-30, 55111-131-37, view more
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
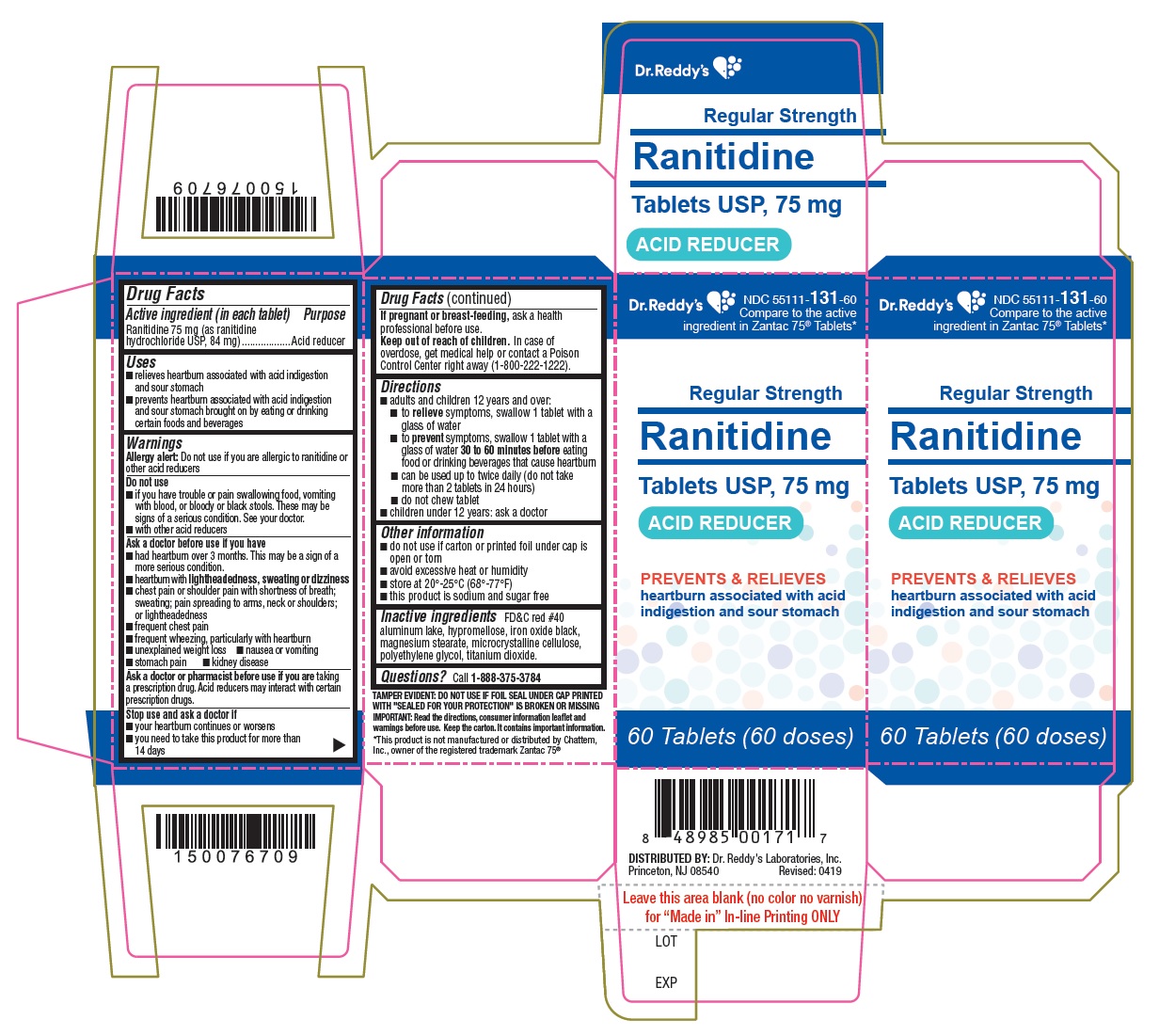
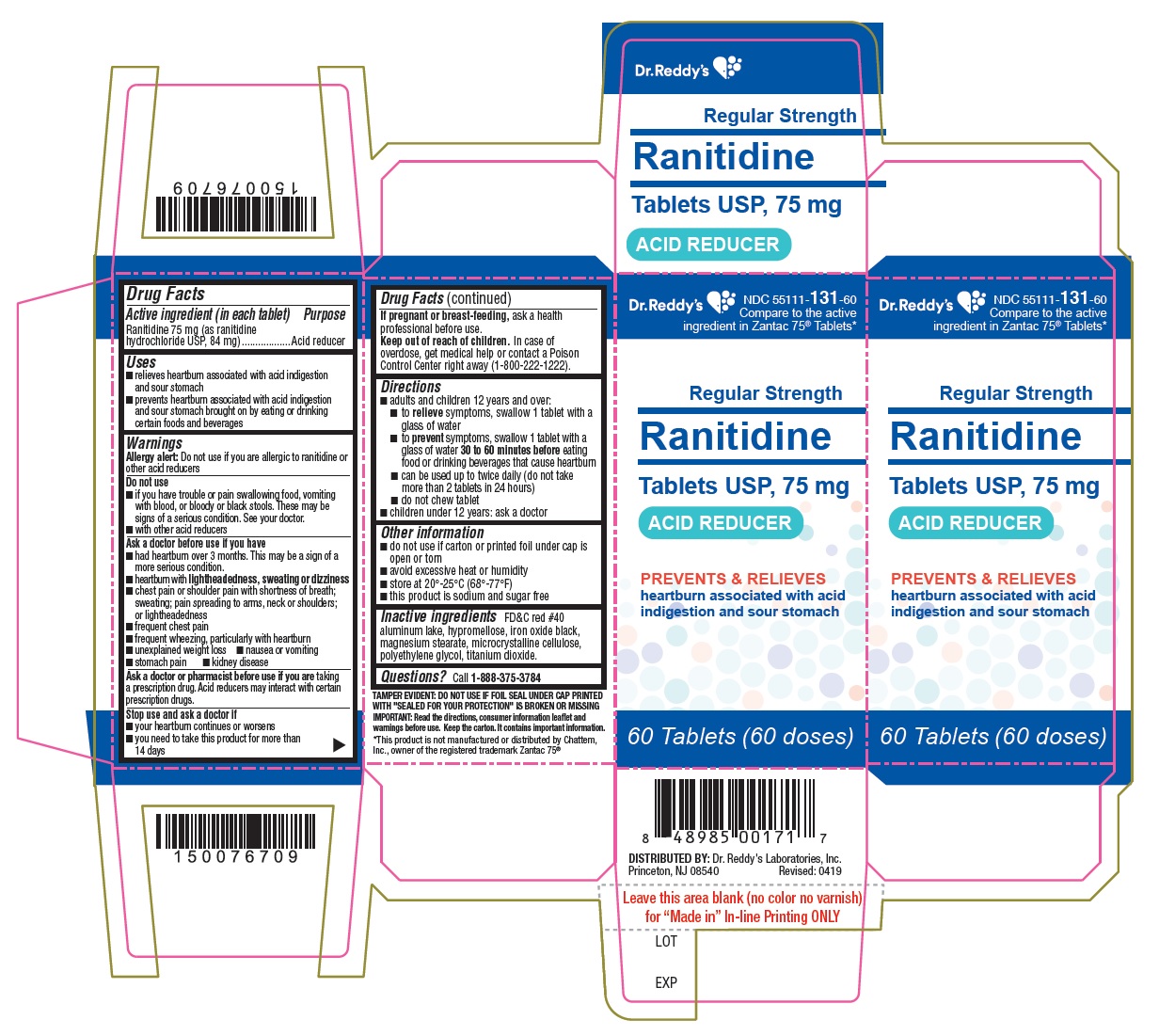
Active ingredient (in each tablet)Ranitidine 75 mg (as ranitidine hydrochloride, USP 84 mg)
-
PurposeAcid reducer
-
Usesrelieves heartburn associated with acid indigestion and sour stomach - prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and ...
-
WarningsAllergy alert: Do not use if you are allergic to ranitidine or other acid reducers - Do not use - if you have trouble or pain swallowing food, vomiting with blood, or bloody or black ...
-
Directionsadults and children 12 years and over: to relieve symptoms, swallow 1 tablet with a glass of water - to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating ...
-
Other informationthis product is sodium and sugar free - Blister: do not use if individual blister unit is open or torn - Bottle: do not use if printed foil under bottle cap is open or torn - avoid excessive heat or ...
-
Inactive ingredientsFD&C red #40 aluminum lake, hypromellose, iron oxide black, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide.
-
QUESTIONSQuestions? call 1-888-375-3784 - Read the directions, consumer information leaflet and warnings before use. Keep the carton. It contains important information.
-
PRINCIPAL DISPLAY PANELRanitidine Tablets USP, 75 mg - container label
-
PRINCIPAL DISPLAY PANELRanitidine Tablets USP, 75 mg - Container CartonLabel
-
INGREDIENTS AND APPEARANCEProduct Information