Label: CLOTRIMAZOLE solution
- NDC Code(s): 70010-229-54
- Packager: Granules Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
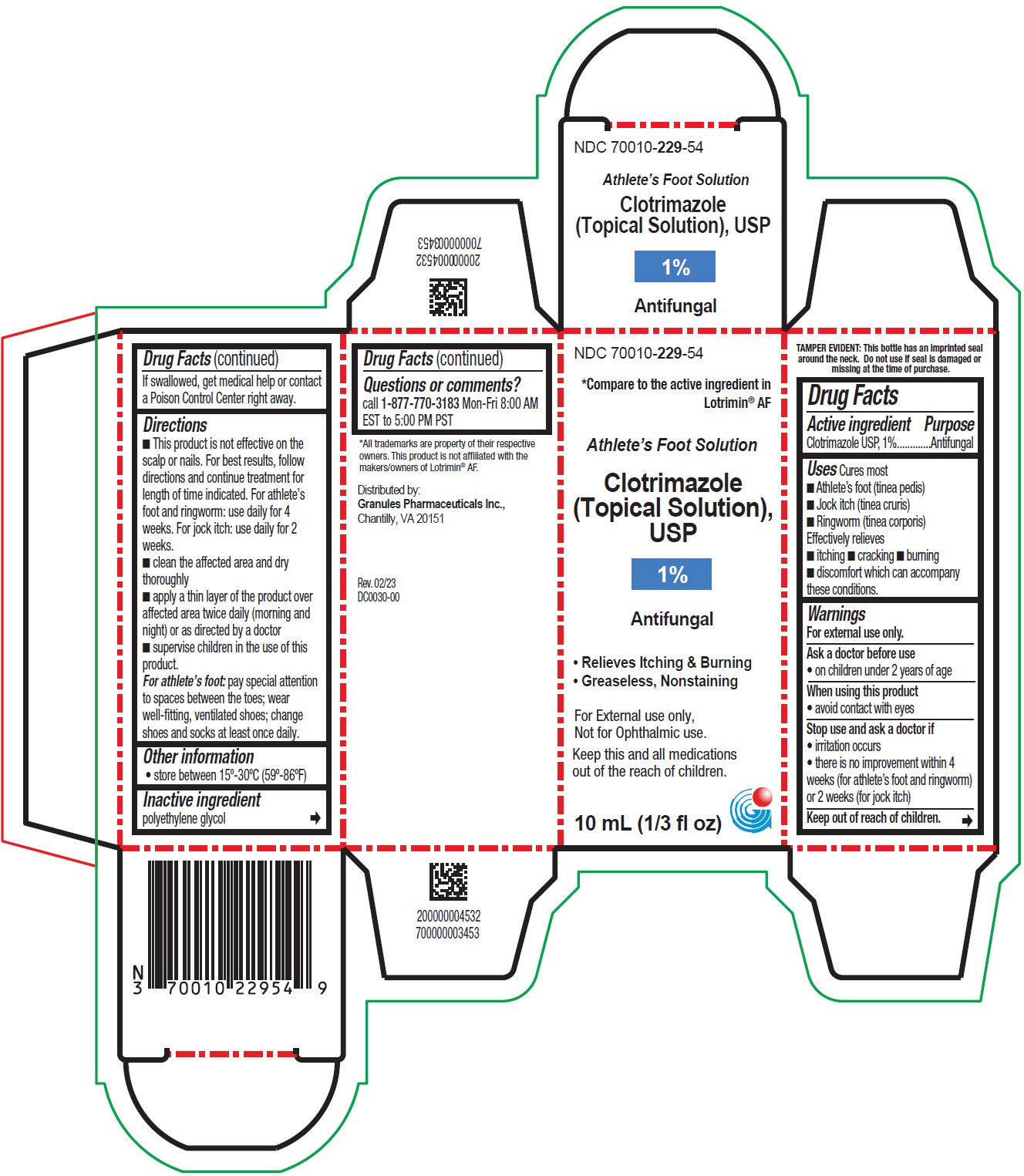
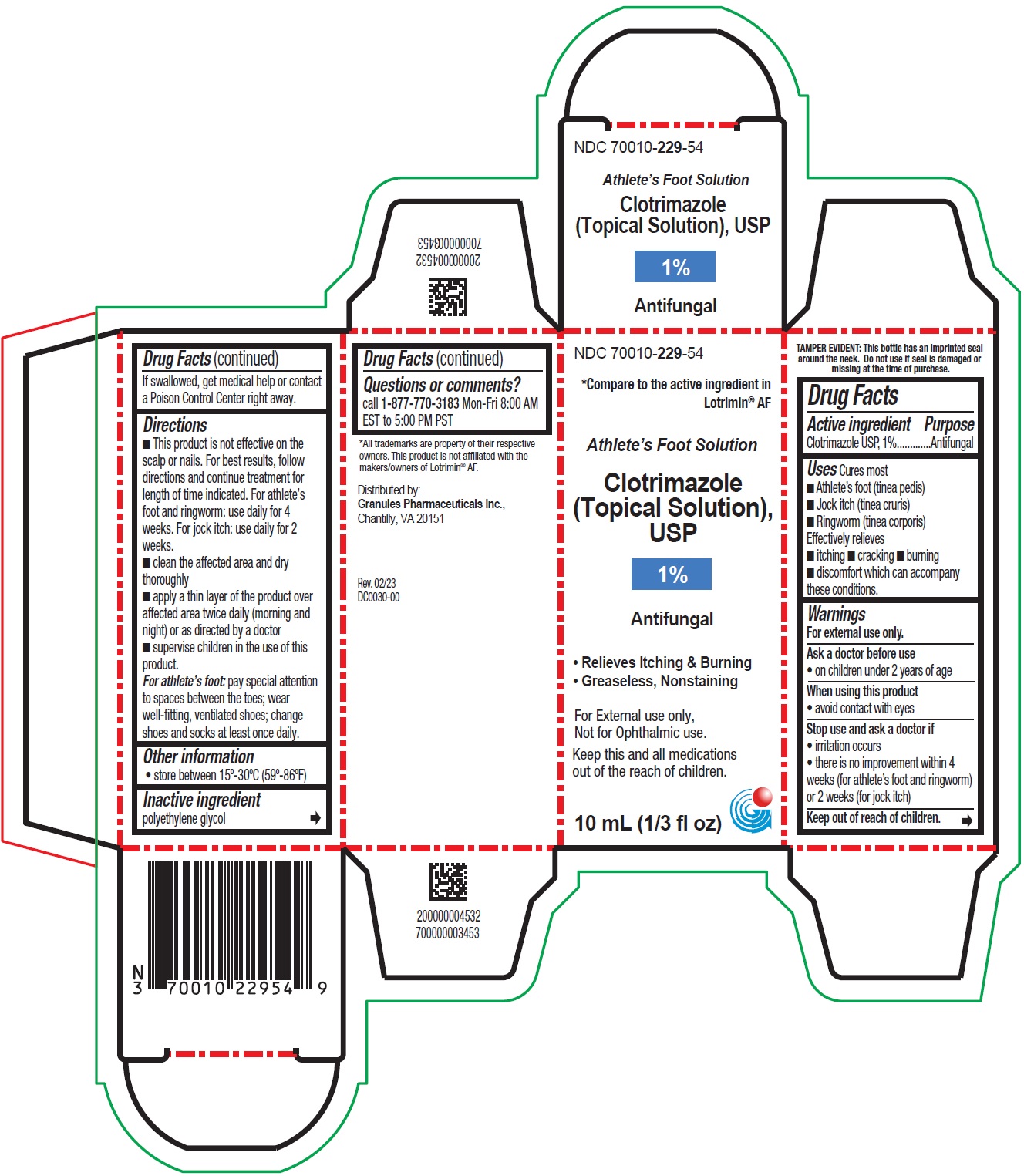
- ■ This product is not effective on the scalp or nails. For best results, follow directions and continue treatment for length of time indicated. For athlete’s foot and ringworm: use daily for 4 weeks. For jock itch: use daily for 2 weeks.
■ clean the affected area and dry thoroughly
■ apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor
■ supervise children in the use of this product.
For athlete’s foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes; change shoes and socks at least once daily.
- ■ This product is not effective on the scalp or nails. For best results, follow directions and continue treatment for length of time indicated. For athlete’s foot and ringworm: use daily for 4 weeks. For jock itch: use daily for 2 weeks.
- Other information
- Inactive ingredient
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - Container Label
-
PRINCIPAL DISPLAY PANEL - Container Carton Label
NDC 70010- 229-54
*Compare to the active ingredient in
Lotrimin ® AF
Athlete's Foot Solution
Clotrimazole
(Topical Solution),
USP
1%
Antifungal
• Relieves Itching & Burning
• Greaseless, Nonstanining
For External use only,
Not for Ophthalmic use.
Keep this and all medications
out of the reach of children.
10 mL (1/3 fl oz)
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70010-229 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70010-229-54 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 03/22/2023 Labeler - Granules Pharmaceuticals Inc. (079825711)