Label: SANINTA 10% POVIDONE-IODINE- povidone-iodine solution liquid
- NDC Code(s): 68356-601-01, 68356-601-02, 68356-601-03
- Packager: Lernapharm
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions or Comments?
-
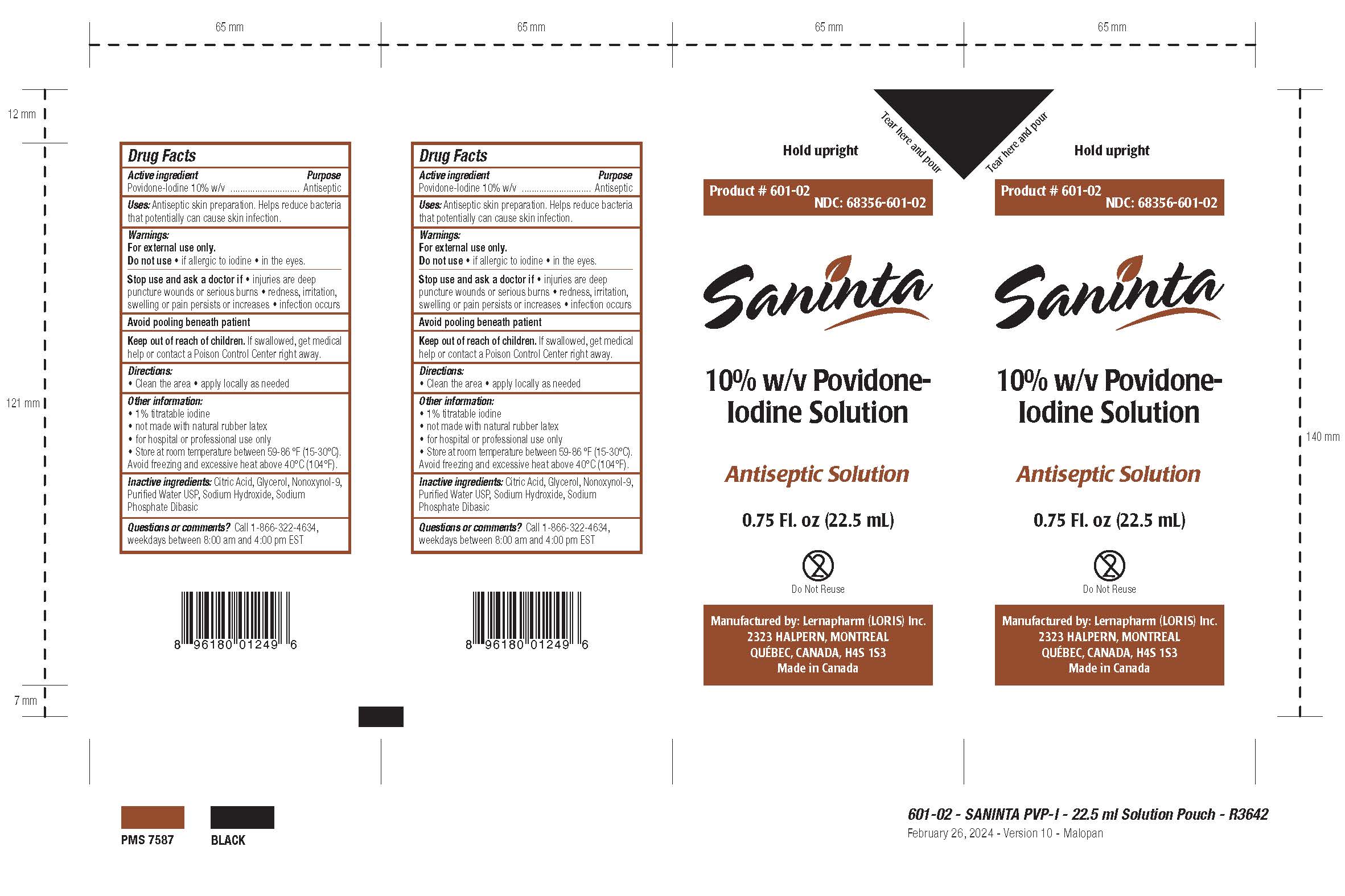
Package Label - Principal Display Panel
NDC: 68356-601-01 / 68356-601-601-02 / 68356-601-03
Product # 601-01 / 601-02 / 601-03
Hold upright
[Tear here and pour]
[Three 4-inch antiseptic swabsticks impregnated with 10% W/V povidone-iodine solution]
[Tear open]
Saninta
Antiseptic Solution
10% w/v Povidone-Iodine Solution
10% Povidone-Iodine
[0.75 Fl. oz (22.5 mL)]
[0.5 Fl. oz (15 mL)]
Do Not Reuse
multiple-use cross-through logo
UPC Code
Manufactured by:
Lernapharm (LORIS) Inc.
2323 HALPERN, MONTREAL
QUEBEC, CANADA H4S 1S3
Made in Canada
LOT #: XXXX
EXP.: MM/YYYY


-
INGREDIENTS AND APPEARANCE
SANINTA 10% POVIDONE-IODINE
povidone-iodine solution liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68356-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) NONOXYNOL-9 (UNII: 48Q180SH9T) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68356-601-01 3 in 1 POUCH 02/26/2024 1 2.3 mL in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:68356-601-02 22.5 mL in 1 PACKET; Type 0: Not a Combination Product 02/26/2024 3 NDC:68356-601-03 15 mL in 1 PACKET; Type 0: Not a Combination Product 11/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/26/2024 Labeler - Lernapharm (206940905) Registrant - Lernapharm (206940905) Establishment Name Address ID/FEI Business Operations Lernapharm 206940905 manufacture(68356-601) , analysis(68356-601) , label(68356-601) , pack(68356-601)