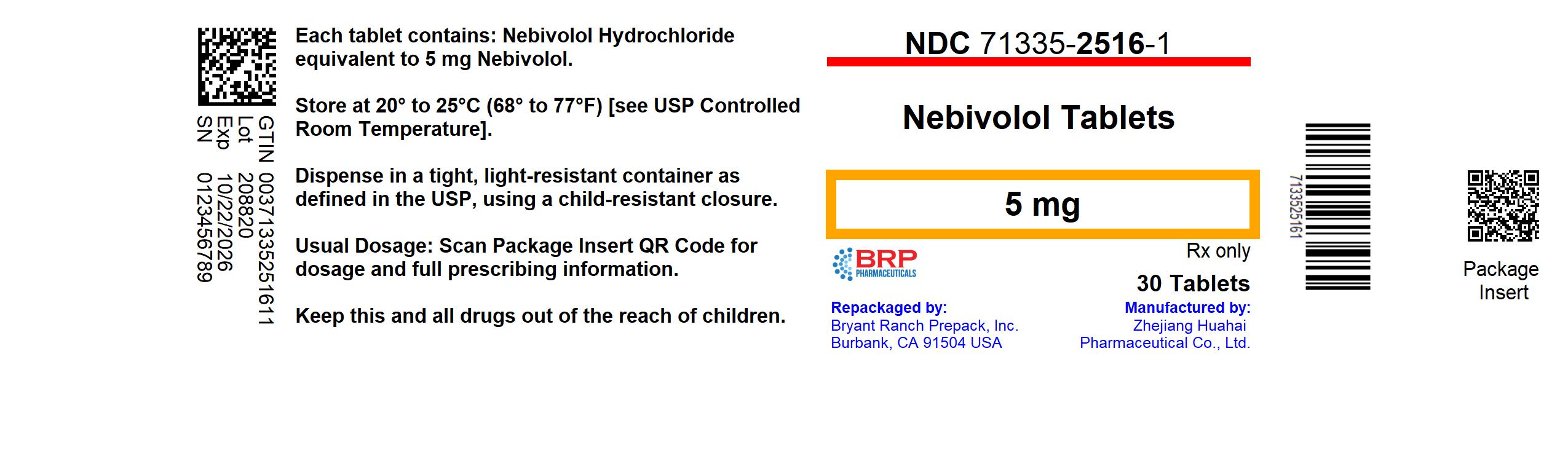
Label: NEBIVOLOL tablet
- NDC Code(s): 71335-2516-1, 71335-2516-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 43547-525
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEBIVOLOL TABLETS safely and effectively. See full prescribing information for NEBIVOLOL TABLETS. NEBIVOLOL tablets, for oral ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGE 1.1 Hypertension Nebivolol tablets are indicated for the treatment of hypertension, to lower blood pressure [see Clinical Studies (14.1)]. Nebivolol tablets may be used alone or in ...
-
2. DOSAGE AND ADMINISTRATION 2.1 Hypertension The dose of nebivolol tablets must be individualized to the needs of the patient. For most patients, the recommended starting dose is 5 mg once daily, with or without food ...
-
3. DOSAGE FORMS AND STRENGTHS Nebivolol tablets are available for oral administration containing nebivolol hydrochloride equivalent to 2.5, 5, 10, and 20 mg of nebivolol. Nebivolol tablets are triangular-shaped, biconvex ...
-
4. CONTRAINDICATIONS Nebivolol tablets are contraindicated in the following conditions: • Severe bradycardia - • Heart block greater than first degree - • Patients with cardiogenic shock - • Decompensated cardiac ...
-
5. WARNINGS AND PRECAUTIONS 5.1 Abrupt Cessation of Therapy Do not abruptly discontinue nebivolol tablets therapy in patients with coronary artery disease. Severe exacerbation of angina, myocardial infarction and ...
-
6. ADVERSE REACTIONS 6.1 Clinical Studies Experience Nebivolol tablets have been evaluated for safety in patients with hypertension and in patients with heart failure. The observed adverse reaction profile was ...
-
7. DRUG INTERACTIONS 7.1 CYP2D6 Inhibitors Use caution when nebivolol tablets are co-administered with CYP2D6 inhibitors (quinidine, propafenone, fluoxetine, paroxetine, etc.) [see Clinical Pharmacology (12.5)] ...
-
8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk Summary - Available data regarding use of nebivolol tablets in pregnant women are insufficient to determine whether there are drug-associated risks of adverse ...
-
10. OVERDOSAGE In clinical trials and worldwide postmarketing experience there were reports of nebivolol tablets overdose. The most common signs and symptoms associated with nebivolol tablets overdosage are ...
-
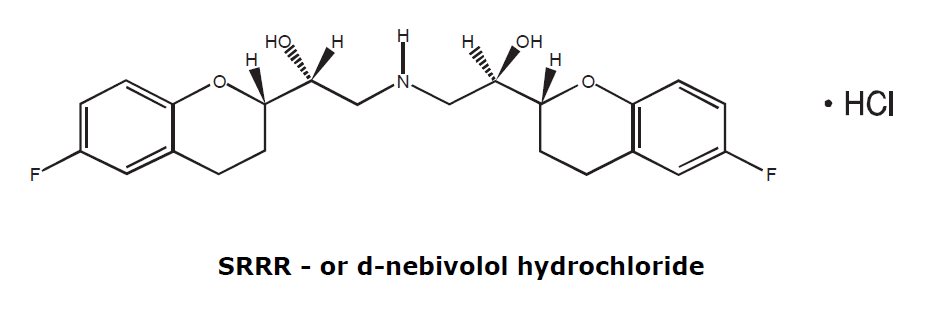
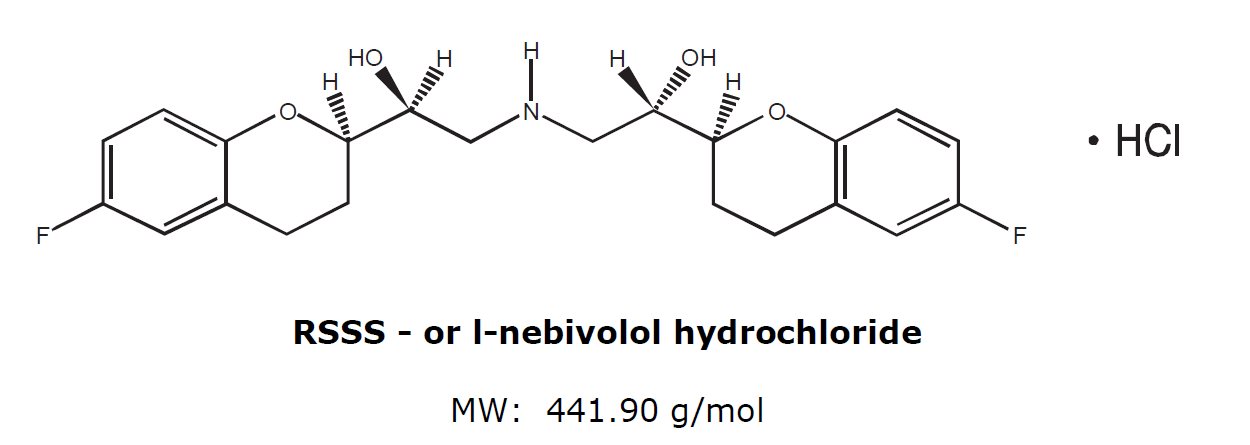
11. DESCRIPTION The chemical name for the active ingredient in nebivolol tablets (nebivolol) tablets is (1RS,1’RS)-1,1’-[(2RS,2’SR)-bis(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)]- 2,2’-iminodiethanol ...
-
12. CLINICAL PHARMACOLOGY Nebivolol is a β-adrenergic receptor blocking agent. In extensive metabolizers (most of the population) and at doses less than or equal to 10 mg, nebivolol is preferentially β1 selective. In poor ...
-
13. NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility In a two-year study of nebivolol in mice, a statistically significant increase in the incidence of testicular Leydig cell hyperplasia ...
-
14. CLINICAL STUDIES 14.1 Hypertension The antihypertensive effectiveness of nebivolol tablets as monotherapy has been demonstrated in three randomized, double-blind, multi-center, placebo-controlled trials at ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING Nebivolol tablets are for oral administration containing nebivolol hydrochloride equivalent to 5 mg of nebivolol. Nebivolol tablets are triangular-shaped, biconvex, unscored, differentiated by ...
-
17. PATIENT COUNSELING INFORMATION See FDA-approved patient labeling (Patient Information). • Patient Advice - Advise patients to take nebivolol tablets regularly and continuously, as directed. Nebivolol tablets can be taken ...
-
PATIENT INFORMATION Dispense with Patient Information available at: www.solcohealthcare.com/druglabeling/nebivolol-tablets.pdf - Nebivolol Tablets - (ne biv′ oh lol) Read the Patient Information that comes with ...
-
PRINCIPAL DISPLAY PANELNebivolol HCl 5mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information