Label: GYNAZOLE 1- butoconazole nitrate cream
- NDC Code(s): 45802-396-01, 45802-396-02
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% contains butoconazole nitrate 2%, an imidazole derivative with antifungal activity. Its chemical name is (±)-1-[4-(p-chlorophenyl)-2- [(2,6-dichlorophenyl) thio]butyl] imidazole mononitrate, and it has the following chemical structure:
Butoconazole nitrate is a white to off-white crystalline powder with a molecular weight of 474.79. It is sparingly soluble in methanol; slightly soluble in chloroform, methylene chloride, acetone, and ethanol; very slightly soluble in ethyl acetate; and practically insoluble in water. It melts at about 159°C with decomposition.
GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% contains 2% butoconazole nitrate in a cream of edetate disodium, glyceryl monoisostearate, methylparaben, mineral oil, polyglyceryl-3 oleate, propylene glycol, propylparaben, colloidal silicon dioxide, sorbitol solution, purified water, and microcrystalline wax.
-
CLINICAL PHARMACOLOGY
Following vaginal administration of butoconazole nitrate vaginal cream, 2% to 3 women, 1.7% (range 1.3-2.2%) of the dose was absorbed on average. Peak plasma levels (13.6-18.6 ng radioequivalents/mL of plasma) of the drug and its metabolites are attained between 12 and 24 hours after vaginal administration.
Microbiology -
The exact mechanism of the antifungal action of butoconazole nitrate is unknown; however, it is presumed to function as other imidazole derivatives via inhibition of steroid synthesis. Imidazoles generally inhibit the conversion of lanosterol to ergosterol, resulting in a change in fungal cell membrane lipid composition. This structural change alters cell permeability and, ultimately, results in the osmotic disruption or growth inhibition of the fungal cell.
Butoconazole nitrate is an imidazole derivative that has fungicidal activity in vitro against Candida spp. and has been demonstrated to be clinically effective against vaginal infections due to Candida albicans. Candida albicans has been identified as the predominant species responsible for vulvovaginal candidiasis.
-
INDICATIONS AND USAGE
GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% is indicated for the local treatment of vulvovaginal candidiasis (infections caused by Candida). The diagnosis should be confirmed by KOH smears and/or cultures (see CLINICAL STUDIES).
Note: GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% is safe and effective in non-pregnant women; however, the safety and effectiveness of this product in pregnant women has not been established (see PRECAUTIONS - Pregnancy).
- CONTRAINDICATIONS
-
CLINICAL STUDIES
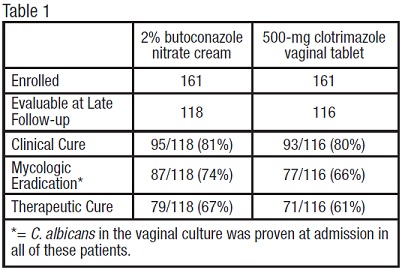
Vulvovaginal Candidiasis: Two studies were conducted that compared 2% butoconazole nitrate cream with clotrimazole tablets. There were 322 enrolled patients, 161 received 2.0% butoconazole vaginal cream and 161 patients inserted the 500-mg clotrimazole vaginal tablet. At the second follow-up visit (30 days post-therapy), 118 patients in the butoconazole group and 116 in the clotrimazole group were evaluable for efficacy analysis, respectively. All of these patients had infection caused by Candida albicans.
The efficacy of the study drugs was assessed by evaluating clinical, mycologic and therapeutic cure rates, which are summarized in Table 1.
The therapeutic cure was defined as complete resolution of signs and symptoms of vaginal candidiasis (clinical cure) along with a negative KOH examination and negative culture for Candida spp. (microbiologic eradication) at the long term follow-up (30 days). The therapeutic cure rate was 67% in the butoconazole group and 61% in the clotrimazole group.
-
WARNINGS
This cream contains mineral oil. Mineral oil may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms; therefore, use of such products within 72 hours following treatment with GYNAZOLE•1® Butoconazole Nitrate Vaginal Cream USP, 2% is not recommended.
Recurrent vaginal yeast infections, especially those that are difficult to eradicate, can be an early sign of infection with the human immunodeficiency virus (HIV) in women who are considered at risk for HIV infection.
-
PRECAUTIONS
General -
If clinical symptoms persist, tests should be repeated to rule out other pathogens, to confirm the original diagnosis, and to rule out other conditions that may predispose a patient to recurrent vaginal fungal infections.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
Carcinogenesis - Long term studies in animals have not been performed to evaluate the carcinogenic potential of this drug.
Mutagenicity - Butoconazole nitrate was not mutagenic when tested in the Ames bacterial test, yeast, chromosomal aberration assay in CHO cells, CHO/HGPRT point mutation assay, mouse micronucleus, and rat dominant lethal assays.
Impairment of Fertility - No impairment of fertility was seen in rabbits or rats administered butoconazole nitrate in oral doses up to 30 mg/kg/day (5 times the human dose based on mg/m2) or 100 mg/kg/day (10 times the human dose based on mg/m2), respectively.
Pregnancy Category C -
In pregnant rats administered 6 mg/kg/day of butoconazole nitrate intravaginally during the period of organogenesis, there was an increase in resorption rate and decrease in litter size; however, no teratogenicity was noted. This dose represents a 130- to 353-fold margin of safety based on serum levels achieved in rats following intravaginal administration compared to the serum levels achieved in humans following intravaginal administration of the recommended therapeutic dose of butoconazole nitrate.
Butoconazole nitrate has no apparent adverse effect when administered orally to pregnant rats throughout organogenesis at dose levels up to 50 mg/kg/day (5 times the human dose based on mg/m2). Daily oral doses of 100, 300 or 750 mg/kg/day (10, 30 or 75 times the human dose based on mg/m2 respectively) resulted in fetal malformations (abdominal wall defects, cleft palate), but maternal stress was also evident at these higher dose levels. There were, however, no adverse effects on litters of rabbits who received butoconazole nitrate orally, even at maternally stressful dose levels (e.g., 150 mg/kg, 24 times the human dose based on mg/m2).
Butoconazole nitrate, like other azole antifungal agents, causes dystocia in rats when treatment is extended through parturition. However, this effect was not apparent in rabbits treated with as much as 100 mg/kg/day orally (16 times the human dose based on mg/m2).
There are, however, no adequate and well-controlled studies in pregnant women. GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
Of the 314 patients treated with GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% for 1 day in controlled clinical trials, 18 patients (5.7%) reported complaints such as vulvar/vaginal burning, itching, soreness and swelling, pelvic or abdominal pain or cramping, or a combination of two or more of these symptoms. In 3 patients (1%) these complaints were considered treatment-related. Five of the 18 patients reporting adverse events discontinued the study because of them.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
Patient Package Insert
GYNAZOLE•1®
Butoconazole Nitrate Vaginal Cream USP, 2%
IN ONE PREFILLED DISPOSABLE APPLICATOR
Using the GYNAZOLE•1® Butoconazole Nitrate Vaginal Cream USP, 2%
Prefilled Disposable Applicator
3 Easy Steps:
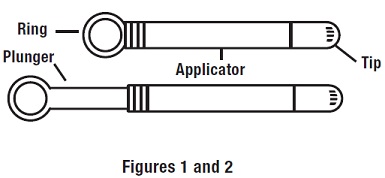
Step 1: Preparing the Applicator
Peel back the protective foil and remove the prefilled applicator. Applicator is designed to be used with tip in place. Do not remove tip; do not use applicator if tip has been removed.
Do not warm applicator before using. While holding the applicator firmly, pull the ring back to fully extend the plunger (see Figures 1 and 2 ).
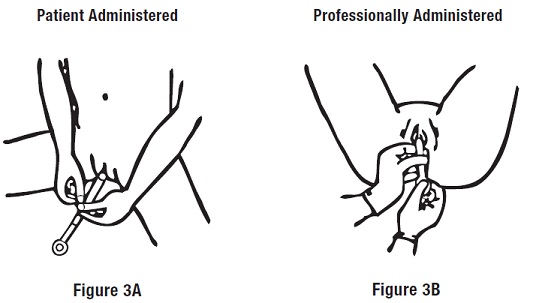
Step 2: Inserting the Applicator
Gently insert the applicator into the vagina as far as it will comfortably go (see Figures 3A and 3B ).
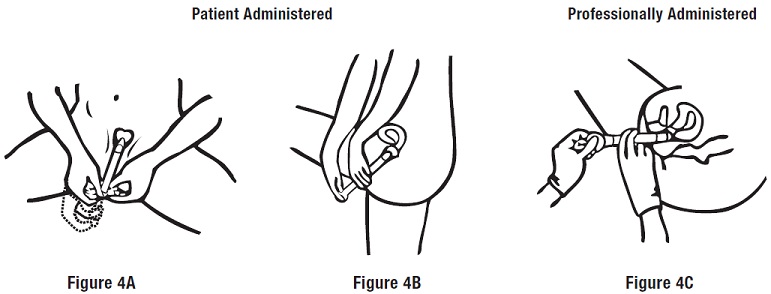
Step 3: Applying the Cream
Push the plunger to release the cream (see Figures 4A, 4B and 4C ). Remove the empty applicator from the vagina and throw it away.
Important Instructions
- •
- One prefilled applicator of GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% should be administered.
- •
- This cream contains mineral oil. Mineral oil may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms; therefore, use of such products within 72 hours following treatment with GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% is not recommended.
- •
- There are no adequate and well-controlled studies in pregnant women. GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% should be used during pregnancy only under the supervision of a physician.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
Manufactured By Padagis
Yeruham, Israel
Distributed By
PadagisTM
Allegan, MI 49010 • www.padagis.com
Rev 05-22
8F200 RC J2
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
NDC 45802-396-01
GYNAZOLE•1®
Butoconazole Nitrate Vaginal Cream USP, 2%
The applicator delivers approximately 5 g cream, containing 100 mg butoconazole nitrate.
IN ONE PREFILLED DISPOSABLE APPLICATOR
For Vaginal Use Only.
NET WT 5.8 g
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
GYNAZOLE 1
butoconazole nitrate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-396 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTOCONAZOLE NITRATE (UNII: 4805237NP5) (BUTOCONAZOLE - UNII:0Q771797PH) BUTOCONAZOLE NITRATE 100 mg in 5 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) POLYGLYCERYL-3 OLEATE (UNII: XRQ165498B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) Product Characteristics Color WHITE (ODORLESS) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-396-01 1 in 1 CARTON 04/29/2015 1 5.8 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC:45802-396-02 1 in 1 CARTON 04/29/2015 2 5.8 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200923 04/29/2015 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)