Label: PROMACE- acepromazine maleate injection
- NDC Code(s): 0010-3827-01
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONApproved by FDA under NADA # 015-030
-
Caution:Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
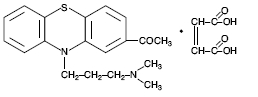
Description:PROMACE Injectable, a potent neuroleptic agent with a low order of toxicity, is of particular value in the tranquilization of dogs, cats and horses. Its rapid action and lack of hypnotic effect ...
-
Indications:Dogs and Cats: PROMACE Injectable can be used as an aid in controlling intractable animals during examination, treatment, grooming, x-ray and minor surgical procedures; to alleviate itching as a ...
-
Dosage and Administration:The dosage should be individualized, depending upon the degree of tranquilization required. As a general rule, the dosage requirement in mg/lb of body weight decreases as the weight of the animal ...
-
Contraindications:Phenothiazines may potentiate the toxicity of organophosphates and the activity of procaine hydrochloride. Therefore, do not use PROMACE Injectable to control tremors associated with organic ...
-
Warning:Do not use in horses intended for human consumption.
-
Precautions:Tranquilizers are potent central nervous system depressants and they can cause marked sedation with suppression of the sympathetic nervous system. Tranquilizers can produce prolonged depression or ...
-
Adverse Reactions:A few rare but serious occurrences of idiosyncratic reactions to acepromazine may occur in dogs following oral or parenteral administration. These potentially serious adverse reactions include ...
-
Animal Safety:Acute and chronic toxicity studies have shown a very low order of toxicity. Acute toxicity: The LD50 dose of PROMACE Injectable in mice was determined by means of a probit transformation with the ...
-
Effectiveness:Controlled clinical studies in the United States and Canada have demonstrated the effectiveness and safety of PROMACE Injectable as a tranquilizer. Good to excellent results were reported1,4,5 in ...
-
Storage:Store at 20° to 25°C (68° to 77°F), excursions permitted between 15° and 30°C (between 59° and 86°F). When used as labeled, there is no limit on the number of punctures throughout the full expiry ...
-
How Supplied:PROMACE Injectable is supplied in 50 mL multiple dose vials. NDC 0010-3827-01 - 10 mg/mL - 50 mL vial
-
References:1. Baker, J.M.: Paper presented at the Ontario Veterinary Association meeting, held in Toronto, Canada,1958. 2. Pharmacology Reports, ClinByla Laboratories, Paris, France. 3. Stegen, M.G. ...
-
SPL UNCLASSIFIED SECTIONPROMACE is a registered trademark of Boehringer Ingelheim Animal Health USA Inc. © 2019 Boehringer Ingelheim Animal Health USA Inc. All rights reserved. Marketed by: Boehringer Ingelheim Animal ...
-
Principal Display Panel – 50 mL Container Label NDC 0010-3827-01 - PromAce® Injectable - (acepromazine maleate injection) 10 mg/mL - Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Net ...
-
INGREDIENTS AND APPEARANCEProduct Information