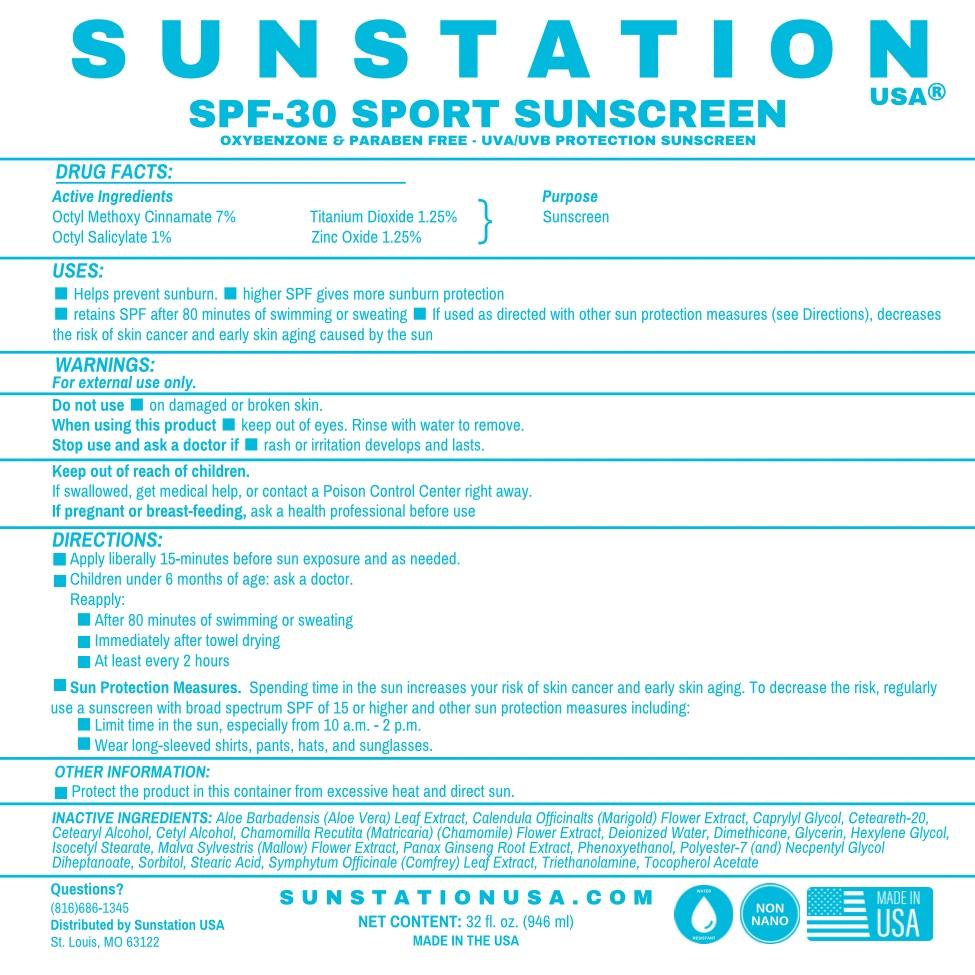
Label: SUNSTATION USA SPF-30 SPORT SUNSCREEN- zinc oxide lotion
- NDC Code(s): 76348-407-01
- Packager: RENU LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- USES
-
WARNINGS
- For external use only.
- DO NOT USE on damaged or broken skin.
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash or irritation develops and lasts
- Keep out of reach of children.
- If swallowed, get medical help, or contact a Poison Control Center right away.
- If pregnant or breast-feeding, ask a health professional before use.
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- After 80 minutes of swimming or sweating.
- Immediately after towel drying.
- At least every 2 hours.
- Sun Protection Measures - Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially between 10am and 2pm.
- Wear long sleeve shirts, pants, hats and sunglasses.
-
OTHER INGREDIENTS
Aloe Barbadensis (Aloe Vera) Leaf Juice, Calendula Officinalis (Marigold) Flower Extract, Caprylyl Glycol, Ceteareth-20, Cetearyl Alcohol, Deionized Water, Dimethicone, Glycerin, Hexylene Glycol, Isocetyl Stearate, Malva Sylvestris (Mallow) Flower Extract, Panax Ginseng Root Extract, Phenoxyethanol, Polyester-7, Neopentyl Glycol, Diheptanoate, Sorbitol, Stearic Acid,
Symphytum Officinale (Comfrey) Leaf Extract, Triethanolamine, Tocopheryl Acetate (Vitamin E)
- STATEMENT OF IDENTITY
- KEEP OUT OF REACH OF CHILDREN
- OTHER SAFETY INFORMATION
- QUESTIONS
- SUNSTATION PRODUCT LABEL
-
INGREDIENTS AND APPEARANCE
SUNSTATION USA SPF-30 SPORT SUNSCREEN
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-407 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 11.2 g in 896 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 63 g in 896 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 9 g in 896 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 11.2 g in 896 g Inactive Ingredients Ingredient Name Strength ASIAN GINSENG (UNII: CUQ3A77YXI) MALVA SYLVESTRIS FLOWER (UNII: 12X9JI52BS) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) MATRICARIA RECUTITA FLOWERING TOP (UNII: 3VNC7T6Z02) COMFREY LEAF (UNII: DG4F8T839X) SORBITOL (UNII: 506T60A25R) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE 350 (UNII: 2Y53S6ATLU) WATER (UNII: 059QF0KO0R) ISOCETYL STEARATE (UNII: 3RJ7186O9W) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYESTER-7 (UNII: 0841698D2F) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) HEXYLENE GLYCOL (UNII: KEH0A3F75J) STEARIC ACID (UNII: 4ELV7Z65AP) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-407-01 896 g in 1 CONTAINER; Type 0: Not a Combination Product 02/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/20/2024 Labeler - RENU LABORATORIES, INC. (945739449) Registrant - RENU LABORATORIES, INC. (945739449) Establishment Name Address ID/FEI Business Operations RENU LABORATORIES, INC. 945739449 manufacture(76348-407)