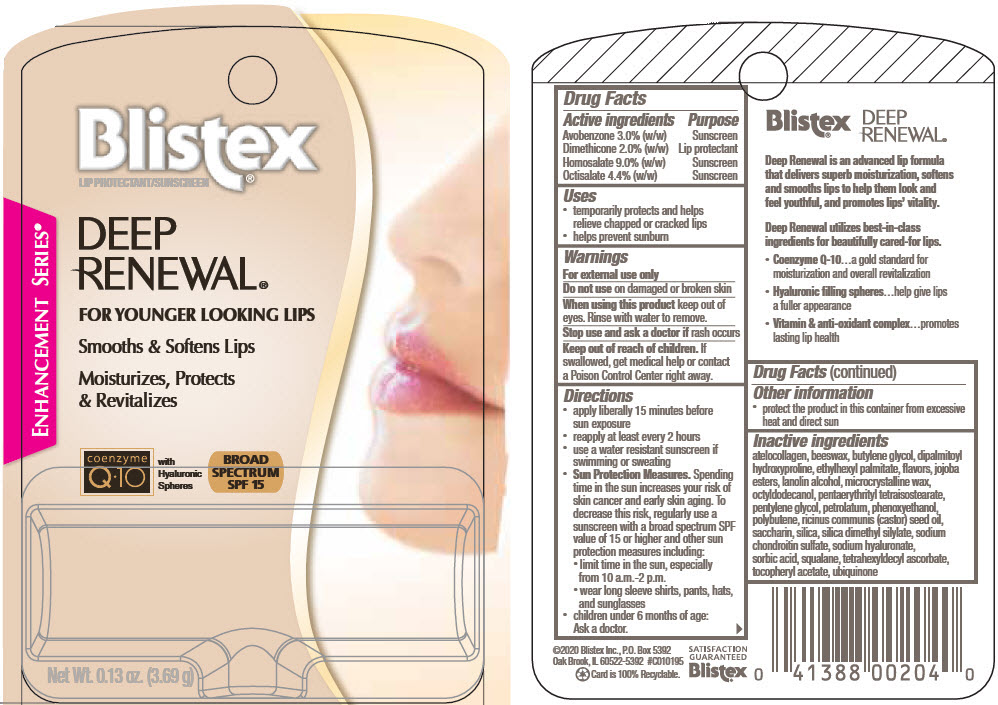
Label: DEEP RENEWAL BROAD SPECTRUM- dimethicone, avobenzone, homosalate, and octisalate stick
- NDC Code(s): 10157-1170-1, 10157-1170-2
- Packager: Blistex Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long sleeve shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor.
- Other information
-
Inactive ingredients
atelocollagen, beeswax, butylene glycol, dipalmitoyl hydroxyproline, ethylhexyl palmitate, flavors, jojoba esters, lanolin alcohol, microcrystalline wax, octyldodecanol, pentaerythrityl tetraisostearate, pentylene glycol, petrolatum, phenoxyethanol, polybutene, ricinus communis (castor) seed oil, saccharin, silica, silica dimethyl silylate, sodium chondroitin sulfate, sodium hyaluronate, sorbic acid, squalane, tetrahexyldecyl ascorbate, tocopheryl acetate, ubiquinone
- PRINCIPAL DISPLAY PANEL - 3.69 g Cylinder Blister Pack
-
INGREDIENTS AND APPEARANCE
DEEP RENEWAL BROAD SPECTRUM
dimethicone, avobenzone, homosalate, and octisalate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10157-1170 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.4 g in 100 g Inactive Ingredients Ingredient Name Strength OCTYLDODECANOL (UNII: 461N1O614Y) PETROLATUM (UNII: 4T6H12BN9U) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CASTOR OIL (UNII: D5340Y2I9G) YELLOW WAX (UNII: 2ZA36H0S2V) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) ETHYLHEXYL PALMITATE (UNII: 2865993309) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) SQUALANE (UNII: GW89575KF9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) CHONDROITIN SULFATE SODIUM, SHARK (UNII: Q75WVO004L) MARINE COLLAGEN, SOLUBLE (UNII: 8JC99XGU4W) PENTYLENE GLYCOL (UNII: 50C1307PZG) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) SACCHARIN (UNII: FST467XS7D) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBIC ACID (UNII: X045WJ989B) UBIDECARENONE (UNII: EJ27X76M46) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10157-1170-1 1 in 1 BLISTER PACK 09/09/2020 1 3.69 g in 1 CYLINDER; Type 0: Not a Combination Product 2 NDC:10157-1170-2 2 in 1 BLISTER PACK 09/09/2020 2 3.69 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/09/2020 Labeler - Blistex Inc (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc 005126354 MANUFACTURE(10157-1170)