Label: DISOPYRAMIDE PHOSPHATE capsule
- NDC Code(s): 0093-3127-01, 0093-3129-01
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
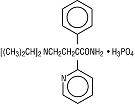
DESCRIPTIONDisopyramide phosphate, USP is an antiarrhythmic drug available for oral administration in capsules containing 100 mg or 150 mg of disopyramide base, present as the phosphate. The base content of ...
-
CLINICAL PHARMACOLOGYMechanisms of Action - Disopyramide phosphate is a Type 1 antiarrhythmic drug (i.e., similar to procainamide and quinidine). In animal studies, disopyramide phosphate decreases the rate of ...
-
INDICATIONS AND USAGEDisopyramide phosphate is indicated for the treatment of documented ventricular arrhythmias such as sustained ventricular tachycardia, that, in the judgment of the physician, are life-threatening ...
-
CONTRAINDICATIONSDisopyramide phosphate is contraindicated in the presence of cardiogenic shock, preexisting second- or third-degree AV block (if no pacemaker is present), congenital Q-T prolongation, or known ...
-
WARNINGSMortality - In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with ...
-
PRECAUTIONSGeneral - Atrial Tachyarrhythmias - Patients with atrial flutter or fibrillation should be digitalized prior to disopyramide phosphate administration to ensure that drug-induced enhancement of AV ...
-
ADVERSE REACTIONSThe adverse reactions which were reported in disopyramide phosphate clinical trials encompass observations in 1,500 patients, including 90 patients studied for at least 4 years. The most serious ...
-
OVERDOSAGESymptoms - Deliberate or accidental overdosage of oral disopyramide may be followed by apnea, loss of consciousness, cardiac arrhythmias, and loss of spontaneous respiration. Death has occurred ...
-
DOSAGE AND ADMINISTRATIONThe dosage of disopyramide phosphate capsules must be individualized for each patient on the basis of response and tolerance. The usual adult dosage of disopyramide phosphate capsules is 400 to ...
-
HOW SUPPLIEDDisopyramide phosphate capsules, USP are supplied as: 100 mg - hard gelatin capsule with a light-blue body imprinted “93-3127” and a scarlet cap imprinted “93-3127”, containing 100 mg of ...
-
Package/Label Display PanelNDC 0093-3127-01 - Disopyramide Phosphate Capsules, USP - 100 mg* Rx only - 100 Capsules
-
Package/Label Display PanelNDC 0093-3129-01 - Disopyramide Phosphate Capsules, USP - 150 mg* Rx only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information