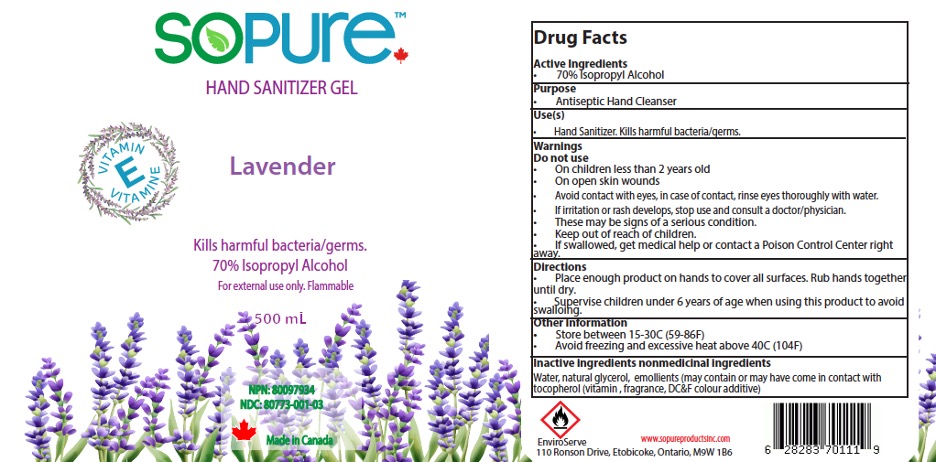
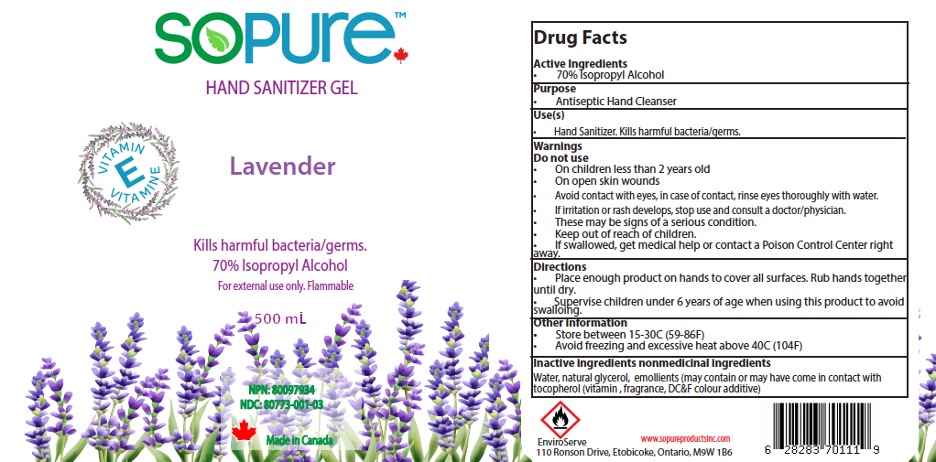
Label: SOPURE HAND SANITIZER GEL- isopropyl alcohol 70% gel hand sanitizer gel
- NDC Code(s): 80773-004-01
- Packager: EnviroServe Chemicals & Cleaners Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- SPL UNCLASSIFIED SECTION
- Purpose
- Uses
-
Warnings
- Flamable. Keep away from fire or flame
- For external use only
- When using this product avoid contact with eyes. In case of eye contact, flush eyes with water
- Stop use and ask a doctor if irritation or rash appears and lasts
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
- Do not use
- When using
- Stop use section
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Package Label- Principal Display Panel
- SoPure hand Sanitizer
-
INGREDIENTS AND APPEARANCE
SOPURE HAND SANITIZER GEL
isopropyl alcohol 70% gel hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80773-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 0.0133 mL in 1 mL CARBOMER 940 (UNII: 4Q93RCW27E) 0.0045 mL in 1 mL AMINOMETHYLPROPANOL (UNII: LU49E6626Q) 0.0035 mL in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.003 mL in 1 mL WATER (UNII: 059QF0KO0R) 0.2747 mL in 1 mL .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) 0.001 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80773-004-01 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/16/2024 Labeler - EnviroServe Chemicals & Cleaners Ltd (243690711) Establishment Name Address ID/FEI Business Operations EnviroServe Chemicals & Cleaners Ltd 243690711 manufacture(80773-004)