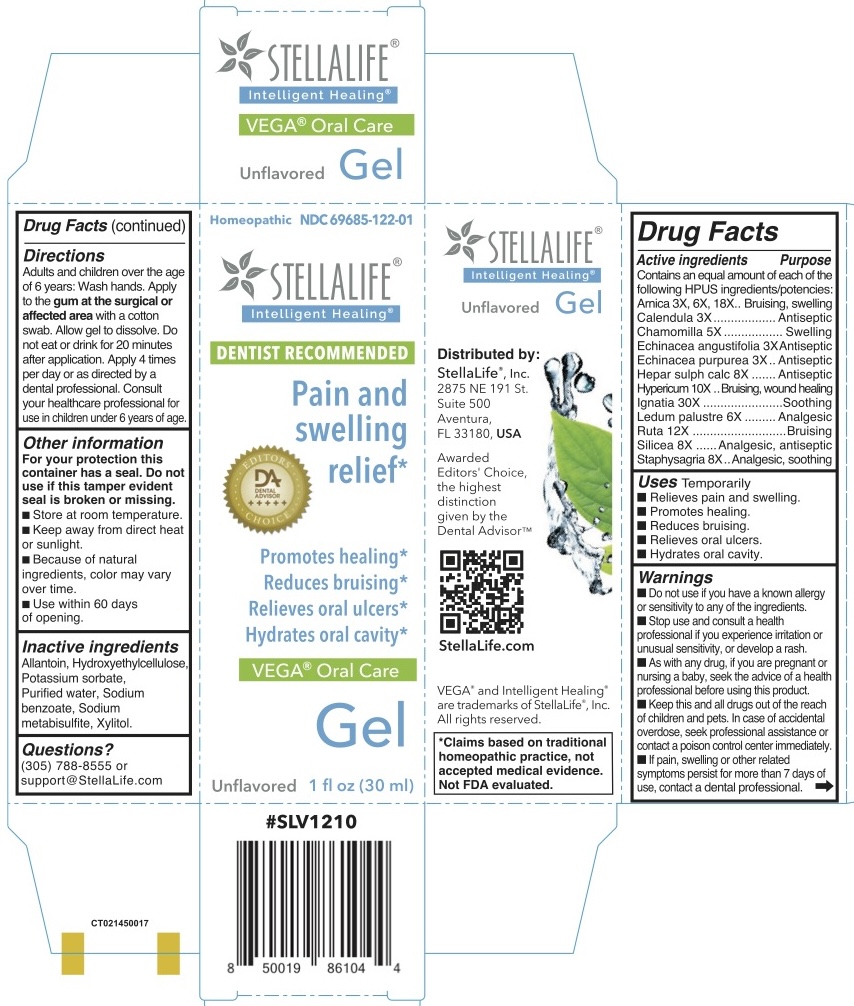
Label: STELLALIFE VEGA ORAL CARE UNFLAVORED PAIN AND SWELLING RELIEF- arnica, calendula, chamomilla, echinacea angustifolia, echinacea purpurea, hepar sulph calc, hypericum, lgnatia, ledum palustre, ruta, silicea, staphysagria gel
- NDC Code(s): 69685-122-01
- Packager: StellaLife, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
-
Purpose
Active Ingredient Purpose Arnica Bruising, swelling Calendula Antiseptic Chamomilla Swelling Echinacea angustifolia Antiseptic Echinacea purpurea Antiseptic Hepar sulph calc Antiseptic Hypericum Bruising, wound healing lgnatia Soothing Ledum palustre Analgesic Ruta Bruising Silicea Analgesic, antiseptic Staphysagria Analgesic, soothing - INDICATIONS & USAGE
-
Directions
Adults and children over the age of 6 years: Wash hands. Apply to the gum at the surgical or affected area with a cotton swab. Allow gel to dissolve. Do not eat or drink for 20 minutes after application. Apply 4 times per day or as directed by a dental professional. Consult your healthcare professional for use in children under6 years of age.
- Keep out of reach of children
- Pregnancy warning
- Inactive ingredients
- Do not use
- Stop use
- Warnings
- Ask dential professional
- Other information
- Questions?
- StellaLife VEGA Oral Care Gel PDP
-
INGREDIENTS AND APPEARANCE
STELLALIFE VEGA ORAL CARE UNFLAVORED PAIN AND SWELLING RELIEF
arnica, calendula, chamomilla, echinacea angustifolia, echinacea purpurea, hepar sulph calc, hypericum, lgnatia, ledum palustre, ruta, silicea, staphysagria gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69685-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 6 [hp_X] in 1 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 1 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 10 [hp_X] in 1 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 8 [hp_X] in 1 mL RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 12 [hp_X] in 1 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 1 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 5 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 3 [hp_X] in 1 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 8 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 8 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) XYLITOL (UNII: VCQ006KQ1E) ALLANTOIN (UNII: 344S277G0Z) SODIUM METABISULFITE (UNII: 4VON5FNS3C) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69685-122-01 1 in 1 BOX 01/08/2024 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/08/2024 Labeler - StellaLife, Inc. (079714251)