Label: SIMETHICONE ENTERIC S.C.- simethicone tablet, sugar coated
- NDC Code(s): 83931-001-01
- Packager: Synmosa Biopharma Corporation Synmosa Plant
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
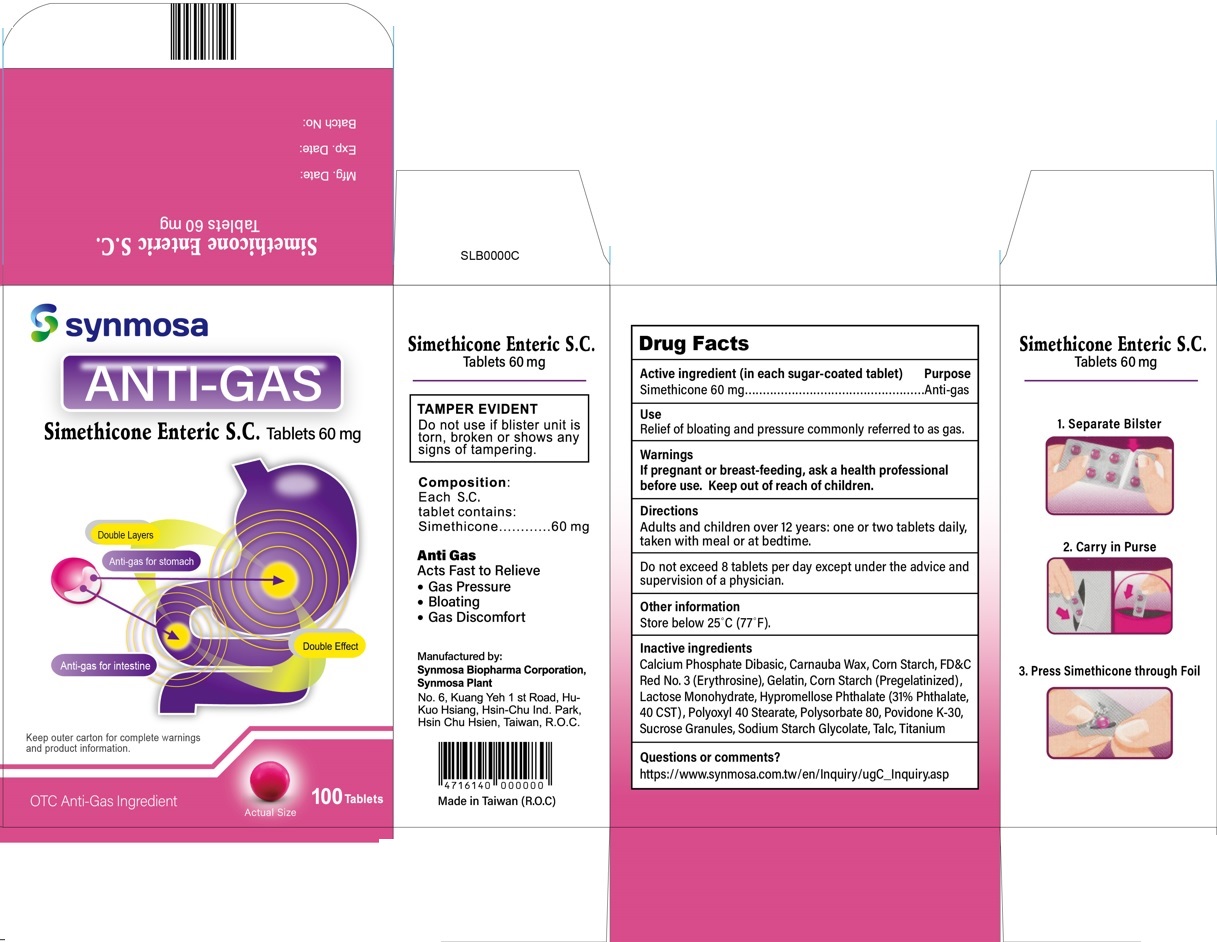
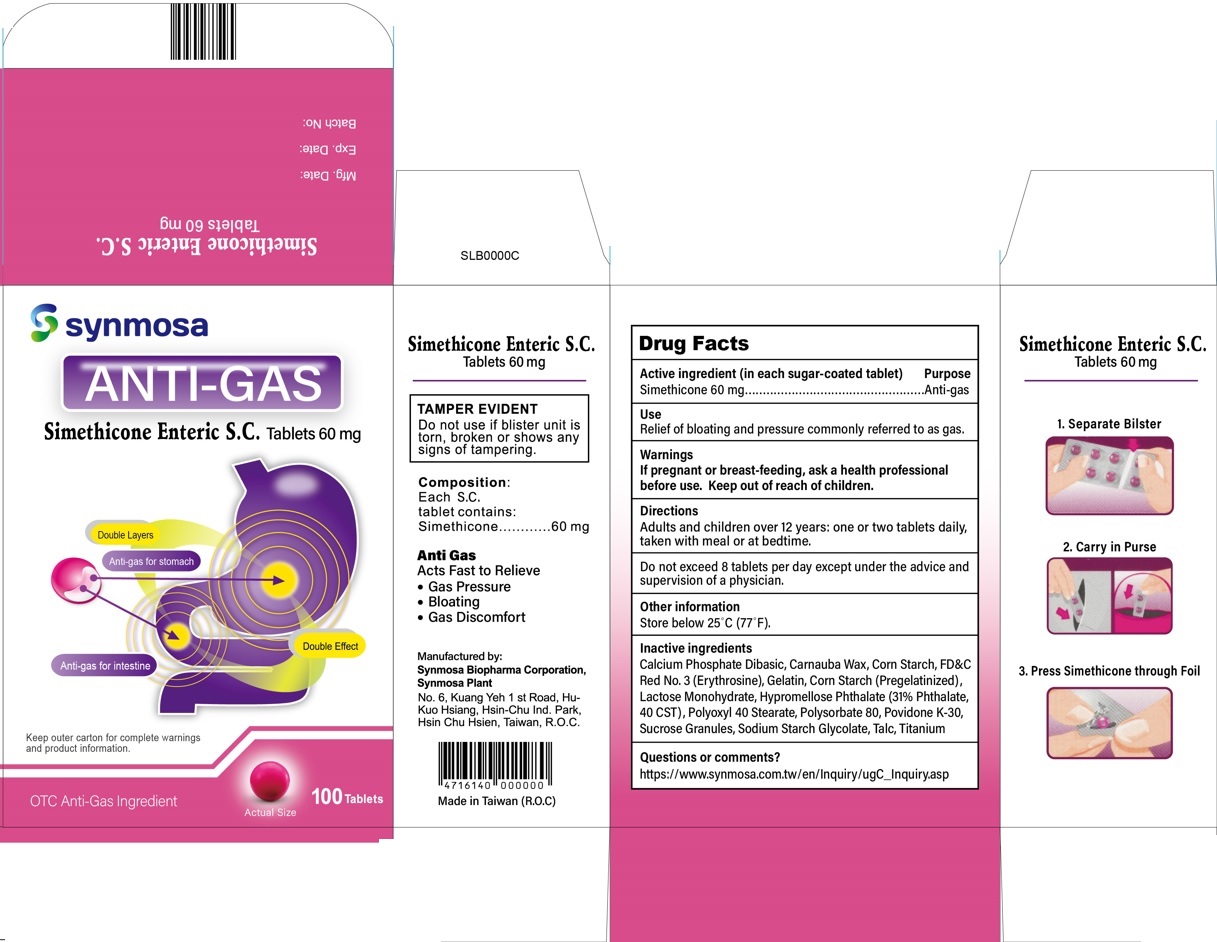
Calcium Phosphate Dibasic, Carnauba Wax, Corn Starch, FD&C Red No. 3 (Erythrosine), Gelatin, Corn Starch (Pregelatinized), Lactose Monohydrate, Hypromellose Phthalate (31% phthalate, 40 CST), Polyoxyl 40 Stearate, Polysorbate 80, Povidone K-30, Sucrose granules, Sodium Starch Glycolate, Talc, Titanium
- Questions or comments?
- OTHER SAFETY INFORMATION
-
SPL UNCLASSIFIED SECTION
Simethicone Enteric S.C.
Tablets 60 mg
Composition:
Each S.C. tablet contains:
Simethicone . . . . . 60 mg
Anti Gas
Acts Fast to Relieve
- Gas Pressure
- Bloating
- Gas Discomfort
Manufactured by:
Synmosa Biopharma Corporation,Synmosa Plant
No. 6, Kuang Yeh 1 st Road, Hu-Kuo Hsiang
Hsin-Chu Ind. Park, Hsin Chu Hsien
Taiwan, R.O.C.
Made in Taiwan (R.O.C.)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SIMETHICONE ENTERIC S.C.
simethicone tablet, sugar coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83931-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 60 mg Inactive Ingredients Ingredient Name Strength POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) HYPROMELLOSE PHTHALATE (31% PHTHALATE, 40 CST) (UNII: G4U024CQK6) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) TITANIUM (UNII: D1JT611TNE) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) CARNAUBA WAX (UNII: R12CBM0EIZ) GELATIN (UNII: 2G86QN327L) Product Characteristics Color pink Score no score Shape ROUND Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83931-001-01 10 in 1 BOX 04/01/2024 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 04/01/2024 Labeler - Synmosa Biopharma Corporation Synmosa Plant (657895530) Establishment Name Address ID/FEI Business Operations Synmosa Biopharma Corporation Synmosa Plant 657895530 manufacture(83931-001)