Label: ZYNYZ- retifanlimab-dlwr injection
- NDC Code(s): 50881-006-03
- Packager: Incyte Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZYNYZ safely and effectively. See full prescribing information for ZYNYZ. ZYNYZ® (retifanlimab-dlwr) injection, for intravenous ...These highlights do not include all the information needed to use ZYNYZ safely and effectively. See full prescribing information for ZYNYZ.
ZYNYZ® (retifanlimab-dlwr) injection, for intravenous use
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ZYNYZ is a programmed death receptor-1 (PD-1)–blocking antibody indicated:
Squamous Cell Carcinoma of the Anal Canal (SCAC)
- in combination with carboplatin and paclitaxel for the first-line treatment of adult patients with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC). (1.1)
- as a single agent for the treatment of adult patients with locally recurrent or metastatic SCAC with disease progression on or intolerance to platinum-based chemotherapy. (1.1)
Merkel Cell Carcinoma (MCC)
- for the treatment of adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). (1.2)
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1.2, 14.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 500 mg/20 mL (25 mg/mL) solution in a single-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Immune-Mediated Adverse Reactions (5.1)
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune‑mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, and immune‑mediated dermatologic adverse reactions, and solid organ transplant rejection.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
- Withhold or permanently discontinue ZYNYZ and administer corticosteroids based on the severity of reaction. (2.2)
- Infusion-Related Reactions: Interrupt, slow the rate of infusion, or permanently discontinue ZYNYZ based on severity of reaction. (2.2, 5.2)
- Complications of Allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD‑1/PD‑L1–blocking antibody. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
ZYNYZ in Combination with Carboplatin and Paclitaxel
- In patients with SCAC, the most common (≥ 20%) adverse reactions are fatigue, peripheral neuropathy, nausea, alopecia, diarrhea, musculoskeletal pain, constipation, hemorrhage, rash, vomiting, decreased appetite, pruritis, and abdominal pain. (6.1)
ZYNYZ as a Single Agent
- In patients with SCAC, the most common (≥ 10%) adverse reactions are fatigue, musculoskeletal pain, diarrhea, non-urinary tract infections, perineal pain, hemorrhage, urinary tract infection, rash, nausea, decreased appetite, constipation, abdominal pain, dyspnea, pyrexia, vomiting, cough, pruritus, hypothyroidism, headache, and decreased weight. (6.1)
- In patients with MCC, the most common (≥ 10%) adverse reactions are fatigue, musculoskeletal pain, pruritus, diarrhea, rash, pyrexia, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Incyte Corporation at 1-855-463-3463 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Squamous Cell Carcinoma of the Anal Canal
1.2 Merkel Cell Carcinoma
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Complications of Allogeneic HSCT
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Squamous Cell Carcinoma of the Anal Canal (SCAC)
14.2 Merkel Cell Carcinoma (MCC)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Squamous Cell Carcinoma of the Anal Canal - ZYNYZ, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of adult patients with inoperable locally ...
1.1 Squamous Cell Carcinoma of the Anal Canal
- ZYNYZ, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of adult patients with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC).
- ZYNYZ, as a single agent, is indicated for the treatment of adult patients with locally recurrent or metastatic SCAC with disease progression on or intolerance to platinum‑based chemotherapy.
Close1.2 Merkel Cell Carcinoma
ZYNYZ is indicated for the treatment of adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC).
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosages of ZYNYZ are provided in Table 1. Administer ZYNYZ as an intravenous infusion after dilution, over 30 minutes, as recommended [see Dosage and ...
2.1 Recommended Dosage
The recommended dosages of ZYNYZ are provided in Table 1.
Administer ZYNYZ as an intravenous infusion after dilution, over 30 minutes, as recommended [see Dosage and Administration (2.3)].
Table 1: Recommended Dosage of ZYNYZ - *
- Refer to the Prescribing Information for the agents administered in combination with ZYNYZ for recommended dosing information, as appropriate.
Indication Recommended Dosage of ZYNYZ Duration of Treatment Combination Therapy* Adult patients with inoperable locally recurrent or metastatic SCAC in combination with carboplatin and paclitaxel 500 mg every 4 weeks Until disease progression, unacceptable toxicity, or up to 12 months Monotherapy Adult patients with locally recurrent or metastatic SCAC with disease progression on or intolerance to platinum-based chemotherapy 500 mg every 4 weeks Until disease progression, unacceptable toxicity, or up to 24 months Adult patients with metastatic or recurrent locally advanced MCC 500 mg every 4 weeks Until disease progression, unacceptable toxicity, or up to 24 months
2.2 Dosage Modifications for Adverse Reactions
No dose reduction of ZYNYZ is recommended. In general, withhold ZYNYZ for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue ZYNYZ for life‑threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone equivalent per day within 12 weeks of initiating steroids.
Dosage modifications for ZYNYZ for adverse reactions that require management different from these general guidelines are summarized in Table 2.
Table 2: Recommended Dosage Modifications for Adverse Reactions AST = aspartate aminotransferase; ALT = alanine aminotransferase; DRESS = drug rash with eosinophilia and systemic symptoms; SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; ULN = upper limit of normal. - *
- Toxicity graded per National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v5.
- †
- Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no resolution within 12 weeks of initiating steroids or inability to reduce prednisone to less than 10 mg/day (or equivalent) within 12 weeks of initiating steroids.
- ‡
- If AST and ALT are less than or equal to ULN at baseline in patients with liver involvement, withhold or permanently discontinue ZYNYZ based on recommendations for hepatitis with no liver involvement.
- §
- Depending on clinical severity, consider withholding for Grade 2 endocrinopathy until symptom improvement with hormone replacement. Resume once acute symptoms have resolved.
Adverse Reaction Severity* ZYNYZ Dosage Modifications
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] Pneumonitis Grade 2 Withhold† Grade 3 or 4 Permanently discontinue Colitis Grade 2 or 3 Withhold† Grade 4 Permanently discontinue Hepatitis with no tumor involvement of the liver
AST or ALT greater than 3 but no more than 8 times ULN
OR
Total bilirubin increases to more than 1.5 and up to 3 times ULNWithhold† AST or ALT increases to more than 8 times ULN
OR
Total bilirubin greater than 3 times ULNPermanently discontinue Hepatitis with tumor involvement of the liver‡ Baseline AST or ALT is more than 1 and up to 3 times ULN and increases more than 5 and up to 10 times ULN
OR
Baseline AST or ALT is more than 3 and up to 5 times ULN and increases more than 8 and up to 10 times ULNWithhold† AST or ALT increases to more than 10 times ULN
OR
Total bilirubin increases to more than 3 times ULNPermanently discontinue Endocrinopathies§
Grade 3 or 4 Withhold until clinically stable or permanently discontinue depending on severity Nephritis with renal dysfunction Grade 2 or 3 increased blood creatinine Withhold† Grade 4 increased blood creatinine Permanently discontinue Exfoliative dermatologic conditions Grade 3 or suspected SJS, TEN, or DRESS Withhold† Grade 4 or confirmed SJS, TEN, or DRESS Permanently discontinue Myocarditis Grade 2, 3, or 4 Permanently discontinue Neurological toxicities Grade 2 Withhold† Grade 3 or 4 Permanently discontinue Other Adverse Reactions Infusion-related reactions [see Warnings and Precautions (5.2)] Grade 1 or 2 Interrupt or slow the rate of infusion Grade 3 or 4
Permanently discontinue Close2.3 Preparation and Administration
Visually inspect the vial for particulate matter and discoloration prior to administration. ZYNYZ is a clear to slightly opalescent, colorless to pale yellow solution and is free of particles. Discard the vial if the solution is cloudy, discolored, or contains particulate matter.
Do not shake the vial.
Preparation
- Withdraw 20 mL (500 mg) of ZYNYZ from one vial and discard vial with any unused portion.
- Dilute ZYNYZ with either 0.9% Sodium Chloride Injection or 5% Dextrose Injection to a final concentration between 1.4 mg/mL and 10 mg/mL. Use polyvinylchloride (PVC) and di-2-ethylhexyl phthalate (DEHP), polyolefin copolymer, polyolefin with polyamide, or ethylene vinyl acetate infusion bags.
- Mix diluted solution by gentle inversion. Do not shake.
- Visually inspect the infusion bag for particulate matter and discoloration prior to administration. Discard if the solution is discolored or contains particulate matter.
Storage of diluted ZYNYZ solution
Protect the diluted ZYNYZ solution from light during storage.
Store diluted ZYNYZ solution:
- At room temperature [up to 25°C (77°F)] for no more than 8 hours from the time of preparation to the end of the infusion.
OR
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours from the time of preparation to the end of the infusion. If refrigerated, allow the diluted solution to come to room temperature prior to administration. The diluted solution must be administered within 4 hours (including infusion time) once it is removed from the refrigerator.
Do not freeze or shake diluted solution.
Administration
- Administer diluted ZYNYZ solution by intravenous infusion over 30 minutes through a polyethylene, polyurethane, or PVC with DEHP intravenous line containing a sterile, non‑pyrogenic, low-protein binding polyethersulfone, polyvinylidene fluoride, or cellulose acetate 0.2 micron to 5 micron in-line or add-on filter or 15 micron mesh in-line or add-on filter. Do NOT administer ZYNYZ as an intravenous push or bolus injection.
- Do not co‑administer other drugs through the same infusion line.
-
3 DOSAGE FORMS AND STRENGTHSInjection: 500 mg/20 mL (25 mg/mL), clear to slightly opalescent, colorless to pale yellow solution in a single-dose vial.
Injection: 500 mg/20 mL (25 mg/mL), clear to slightly opalescent, colorless to pale yellow solution in a single-dose vial.
Close
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Severe and Fatal Immune-Mediated Adverse - Reactions - ZYNYZ is a monoclonal antibody that belongs to a class of drugs that binds to either the programmed death receptor-1 (PD-1) or the ...
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
ZYNYZ is a monoclonal antibody that belongs to a class of drugs that binds to either the programmed death receptor-1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response with the potential for breaking of peripheral tolerance and induction of immune-mediated adverse reactions. Important immune‑mediated adverse reactions listed under Warnings and Precautions may not be inclusive of all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1–blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1–blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1–blocking antibodies. Immune-mediated adverse reactions affecting more than one body system can occur simultaneously.
Early identification and management of immune‐mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1–blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue ZYNYZ depending on severity [see Dosage and Administration (2.2)]. In general, if ZYNYZ requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroids.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
ZYNYZ can cause immune-mediated pneumonitis. In patients treated with other PD-1/PD-L1–blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-mediated pneumonitis occurred in 3% (13/440) of patients receiving ZYNYZ, including 1 (0.2%) patient with fatal pneumonitis, Grade 3 (0.9%), and Grade 2 (1.4%). Pneumonitis led to permanent discontinuation of ZYNYZ in 1 patient and withholding of ZYNYZ in 0.9% of patients.
Systemic corticosteroids were required in 77% (10/13) of patients with pneumonitis. Pneumonitis resolved in 10 of the 13 patients. Of the 4 patients in whom ZYNYZ was withheld for pneumonitis, 3 reinitiated ZYNYZ after symptom improvement; of these, 1 had recurrence of pneumonitis.
Immune-Mediated Colitis
ZYNYZ can cause immune-mediated colitis. Cytomegalovirus infection/reactivation have occurred in patients with corticosteroid-refractory immune-mediated colitis treated with PD-1/PD-L1–blocking antibodies. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
ZYNYZ as a Single Agent: Immune-mediated colitis occurred in 1.6% (7/440) of patients receiving ZYNYZ, including Grade 4 (0.2%), Grade 3 (0.2%), and Grade 2 (0.7%). Colitis led to permanent discontinuation of ZYNYZ in 1 patient and withholding of ZYNYZ in 0.9% of patients.
Systemic corticosteroids were required in 71% (5/7) of patients. Colitis resolved in 4 of the 7 patients. Of the 4 patients in whom ZYNYZ was withheld for colitis, 1 reinitiated ZYNYZ after symptom improvement; this patient did not have recurrence of colitis.
ZYNYZ in Combination with Carboplatin and Paclitaxel: Immune-mediated colitis occurred in 10% (16/154) of patients receiving ZYNYZ in combination with carboplatin and paclitaxel, including Grade 4 (0.6%), Grade 3 (2.6%), and Grade 2 (3.2%). Colitis led to permanent discontinuation of ZYNYZ in 2 patients and withholding of ZYNYZ in 2 patients. Systemic corticosteroids were required in 94% (15/16) of patients. Colitis resolved in 15 of the 16 patients. Of the 2 patients in whom ZYNYZ was withheld for colitis, both reinitiated ZYNYZ after symptom improvement; neither patient had a recurrence of colitis.
Immune-Mediated Hepatitis
ZYNYZ can cause immune-mediated hepatitis.
Immune-mediated hepatitis occurred in 3% (13/440) of patients receiving ZYNYZ, including Grade 4 (0.2%), Grade 3 (2.3%), and Grade 2 (0.5%). Hepatitis led to permanent discontinuation of ZYNYZ in 1.4% of patients and withholding of ZYNYZ in 0.9% of patients.
Systemic corticosteroids were required in 85% (11/13) of patients. Hepatitis resolved in 6 of the 13 patients. Of the 4 patients in whom ZYNYZ was withheld for hepatitis, 2 reinitiated ZYNYZ after symptom improvement; of these, 1 had recurrence of hepatitis.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
ZYNYZ can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment per institutional guidelines, including hormone replacement as clinically indicated. Withhold or permanently discontinue ZYNYZ depending on severity [see Dosage and Administration (2.2)].
ZYNYZ as a Single Agent: Adrenal insufficiency occurred in 0.7% (3/440) of patients receiving ZYNYZ, including Grade 3 (0.5%) and Grade 2 (0.2%). Adrenal insufficiency did not lead to permanent discontinuation of ZYNYZ. ZYNYZ was withheld for 1 patient with adrenal insufficiency. All patients required systemic corticosteroids. Adrenal insufficiency resolved in 1 of the 3 patients.
ZYNYZ in Combination with Carboplatin and Paclitaxel: Adrenal insufficiency occurred in 5.8% (9/154) of patients receiving ZYNYZ in combination with carboplatin and paclitaxel, including Grade 3 and Grade 2 (1.9% each). Adrenal insufficiency led to permanent discontinuation of ZYNYZ in 1 patient and withholding of ZYNYZ in 3 patients. All patients required systemic corticosteroids. Adrenal insufficiency resolved in 4 of the 9 patients.
Hypophysitis
ZYNYZ can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue ZYNYZ depending on severity [see Dosage and Administration (2.2)].
Hypophysitis occurred in 0.5% (2/440, both Grade 2) of patients receiving ZYNYZ. No patients discontinued or withheld ZYNYZ due to hypophysitis. All patients required systemic steroids. Hypophysitis resolved in 1 of the 2 patients.
Thyroid Disorders
ZYNYZ can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement or medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue ZYNYZ depending on severity [see Dosage and Administration (2.2)].
Thyroiditis occurred in 0.7% (3/440, all Grade 1) of patients receiving ZYNYZ. No patients discontinued or withheld ZYNYZ due to thyroiditis. Thyroiditis resolved in 1 of the 3 patients.
Hypothyroidism
Hypothyroidism occurred in 10% (42/440) of patients receiving ZYNYZ, including Grade 2 (4.8%). No patients discontinued ZYNYZ due to hypothyroidism. Hypothyroidism led to withholding of ZYNYZ in 0.5% of patients. Systemic corticosteroids were required for 1 patient and 79% (33/42) of patients received endocrine therapy.
Hyperthyroidism
Hyperthyroidism occurred in 6% (24/440) of patients receiving ZYNYZ, including Grade 2 (2.5%). No patients discontinued ZYNYZ due to hyperthyroidism. Hyperthyroidism led to withholding of ZYNYZ in 1 patient. Systemic corticosteroids were required for 13% (3/24) of patients and 46% (11/24) of patients received endocrine therapy.
Type 1 Diabetes Mellitus, Which Can Present with Diabetic Ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold ZYNYZ depending on severity [see Dosage and Administration (2.2)].
Type 1 diabetes mellitus occurred in 0.2% (1/440) of patients receiving ZYNYZ, including Grade 3 (0.2%) adverse reactions. Type 1 diabetes mellitus led to withholding of ZYNYZ in 1 patient. This event led to ZYNYZ being withheld and did not lead to permanent discontinuation of ZYNYZ. The patient received insulin.
Immune-Mediated Nephritis with Renal Dysfunction
ZYNYZ can cause immune-mediated nephritis.
Immune-mediated nephritis occurred in 1.6% (7/440) of patients receiving ZYNYZ, including Grade 4 (0.5%), Grade 3 (0.7%), and Grade 2 (0.5%). Nephritis led to permanent discontinuation of ZYNYZ in 0.9% of patients and withholding of ZYNYZ in 1 patient.
Systemic corticosteroids were required in 57% (4/7) of patients. Nephritis resolved in 3 of the 7 patients. The 1 patient in whom ZYNYZ was withheld for immune-mediated nephritis had ZYNYZ reinitiated after symptom improvement and did not have recurrence of immune-mediated nephritis.
Immune-Mediated Dermatologic Adverse Reactions
ZYNYZ can cause immune-mediated rash or dermatitis. Bullous and exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1–blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue ZYNYZ depending on severity [see Dosage and Administration (2.2)].
Immune-mediated skin reactions occurred in 8% (36/440) of patients receiving ZYNYZ, including Grade 3 (1.1%) and Grade 2 (7%). Immune-mediated dermatologic adverse reactions led to permanent discontinuation of ZYNYZ in 1 patient and withholding of ZYNYZ in 2.3% of patients.
Systemic corticosteroids were required in 25% (9/36) of patients. Immune-mediated dermatologic adverse reactions resolved in 75% (27/36) of patients. Of the 10 patients in whom ZYNYZ was withheld for immune-mediated dermatologic adverse reactions, 7 reinitiated ZYNYZ after symptom improvement; of these, 1 had recurrence of immune-mediated dermatologic adverse reactions.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of < 1% in 440 patients who received ZYNYZ [see Adverse Reactions (6.1)] or were reported with the use of other PD-1/PD-L1–blocking antibodies, including severe or fatal cases.
Cardiac/vascular: myocarditis, pericarditis, vasculitis
Gastrointestinal: pancreatitis, to include increases in serum amylase and lipase levels, gastritis, duodenitis
Musculoskeletal: myositis/polymyositis, rhabdomyolysis (and associated sequelae, including renal failure), arthritis, polymyalgia rheumatica
Neurological: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy
Ocular: uveitis, iritis, and other ocular inflammatory toxicities. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt‑Koyanagi-Harada–like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Endocrine: hypoparathyroidism
Other (Hematologic/Immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection, other transplant (including corneal graft) rejection.
5.2 Infusion-Related Reactions
A severe infusion-related reaction (Grade 3) occurred in 4 (0.7%) of 594 patients receiving ZYNYZ [see Adverse Reactions (6.1)]. Monitor patients for signs and symptoms of infusion‑related reactions. Interrupt or slow the rate of infusion or permanently discontinue ZYNYZ based on severity of reaction [see Dosage and Administration (2.2)]. Consider premedication with an antipyretic and/or an antihistamine for patients who have had previous systemic reactions to infusions of therapeutic proteins.
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1–blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1–blocking antibody prior to or after an allogeneic HSCT.
Close5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, ZYNYZ can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus, resulting in fetal death. Advise women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZYNYZ and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in the labeling. Severe and Fatal Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] Infusion-Related Reactions [see ...
The following adverse reactions are described elsewhere in the labeling.
- Severe and Fatal Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Complications of Allogeneic HSCT [see Warnings and Precautions (5.3)]
Close6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population described in Warnings and Precautions reflects exposure to ZYNYZ 500 mg as an intravenous infusion every 4 weeks in combination with carboplatin and paclitaxel in 154 patients with SCAC enrolled in the POD1UM-303 trial, and as a single agent in 94 patients with SCAC in the POD1UM-202 trial, 105 patients with MCC in the POD1UM-201 trial, and 241 patients with other solid tumors. All patients received ZYNYZ until disease progression or unacceptable toxicity; those in the POD1UM-202 and POD1UM-201 trials received ZYNYZ for up to 24 months and those in the POD1UM-303 trial received ZYNYZ for up to 12 months. The median duration of exposure of the pooled monotherapy population was 4.6 months (range: 1 day to 27 months).
Squamous Cell Carcinoma of the Anal Canal (SCAC)
Inoperable Locally Recurrent or Metastatic SCAC: ZYNYZ in Combination with Carboplatin and Paclitaxel
The safety of ZYNYZ in patients with inoperable locally recurrent or metastatic SCAC was evaluated in 154 patients enrolled in the POD1UM-303 trial [see Clinical Studies (14.1)]. Patients received ZYNYZ 500 mg or placebo intravenously every 4 weeks in combination with carboplatin and paclitaxel for 6 cycles followed by ZYNYZ 500 mg or placebo every 4 weeks until disease progression or unacceptable toxicity. Among patients who received ZYNYZ, the median duration of exposure was 7.4 months (range: 1 day to 14.6 months).
Serious adverse reactions occurred in 47% of patients receiving ZYNYZ in combination with carboplatin and paclitaxel. The most frequent serious adverse reactions (≥ 2% of patients) were sepsis (3.2%), pulmonary embolism (3.2%), diarrhea (2.6%), and vomiting (2.6%).
In patients receiving ZYNYZ in combination with carboplatin and paclitaxel, ZYNYZ was permanently discontinued due to an adverse reaction in 11% of patients. Adverse reactions that resulted in permanent discontinuation of ZYNYZ included immune-mediated enterocolitis (2 patients), warm autoimmune hemolytic anemia, hepatitis, adrenal insufficiency, blood bilirubin increased, AST increased, blood alkaline phosphatase increased, arthritis, encephalopathy, peripheral sensorimotor neuropathy, hypothyroidism, immune‑mediated cholangitis, pruritus, malaise, and rash (1 patient each).
Dosage interruptions due to an adverse reaction, excluding temporary interruptions due to infusion-related reactions, occurred in 55% of patients who received ZYNYZ in combination with carboplatin and paclitaxel. Adverse reactions that resulted in dosage interruptions in ≥ 2% of patients were neutropenia, anemia, thrombocytopenia, leukopenia, fatigue, COVID-19, and urinary tract infection.
The most common (≥ 20%) adverse reactions were fatigue, peripheral neuropathy, nausea, alopecia, diarrhea, musculoskeletal pain, constipation, hemorrhage, rash, vomiting, decreased appetite, pruritus, and abdominal pain.
Table 3 and Table 4 summarize adverse reactions and laboratory abnormalities, respectively, that occurred in POD1UM-303.
Table 3: Adverse Reactions in ≥ 10% of Patients with Inoperable Locally Recurrent or Metastatic SCAC Receiving ZYNYZ in Combination with Carboplatin and Paclitaxel with a Difference Between Arms of ≥ 5% for All Grades or ≥ 2% for Grades 3 or 4 vs Placebo in Combination with Carboplatin and Paclitaxel in POD1UM-303 - *
-
Includes diarrhea, colitis, and frequent bowel movements.
- †
-
Includes stomatitis, aphthous ulcer, cheilitis, mouth ulceration, and mucosal inflammation.
- ‡
- Includes peripheral neuropathy, paresthesia, peripheral sensory neuropathy, neuralgia, hypoesthesia, peripheral sensorimotor neuropathy, dysesthesia, peripheral motor neuropathy, and hyperesthesia.
- §
- Includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, pain in extremity, and spinal pain.
- ¶
- Includes hemorrhage, anal hemorrhage, anal ulcer hemorrhage, conjunctival hemorrhage, epistaxis, gastrointestinal hemorrhage, genital hemorrhage, hematuria, hemoptysis, hemorrhoidal hemorrhage, lower gastrointestinal hemorrhage, lymph node hemorrhage, rectal hemorrhage, stoma site hemorrhage, tumor hemorrhage, urinary bladder hemorrhage, uterine hemorrhage, vaginal hemorrhage, and wound hemorrhage.
- #
- Includes rash, eczema, dermatitis acneiform, dermatitis, rash erythematous, rash maculo-papular, rash papular, rash pustular, and rash pruritic.
Adverse Reaction ZYNYZ in Combination with Carboplatin and Paclitaxel
(N = 154)Placebo in Combination with Carboplatin and Paclitaxel
(N = 152)All Grades (%) Grades 3-4
(%)All Grades (%) Grades 3-4
(%)Gastrointestinal disorders Diarrhea* 49 5 41 7 Stomatitis† 18 0 11 0 Nervous system disorders Peripheral neuropathy‡ 56 5 52 2.6 Musculoskeletal and connective tissue disorders Musculoskeletal pain§ 40 2.6 34 0 Vascular disorders Hemorrhage¶ 29 3.2 21 0 Skin and subcutaneous tissue disorders Rash# 29 1.3 16 0.7 Pruritus 24 0.6 7 0 Endocrine disorders Hypothyroidism 14 0.6 3.3 0 Graded according to NCI CTCAE v5.0. Table 4: Laboratory Abnormalities that Worsened from Baseline to Grade 3 or 4 Occurring in ≥ 1% of Patients with Inoperable Locally Recurrent or Metastatic SCAC Receiving ZYNYZ in Combination with Carboplatin and Paclitaxel in POD1UM-303 - *
- The denominator used to calculate the rate varied from 142 to 153 based on the number of patients with a baseline value and at least one post-treatment value.
Laboratory abnormality ZYNYZ in Combination with Carboplatin and Paclitaxel* Placebo in Combination with Carboplatin and Paclitaxel* All Grades (%) Grades 3-4 (%) All Grades (%) Grades 3-4 (%) Hematology Decreased hemoglobin 91 20 92 24 Decreased leukocytes
88 44 90 40 Decreased neutrophils 79 52 78 39 Decreased lymphocytes 77 40 69 38 Decreased platelets 55 6 52 4 Chemistry Decreased albumin 37 2 29 1.3 Increased alanine aminotransferase 35 3.9 23 0.7 Increased aspartate aminotransferase 26 3.9 16 0 Increased alkaline phosphatase 26 2 25 0 Decreased potassium 24 5 16 2.6 Increased calcium 23 2 20 1.3 Increased lipase 18 4.9 15 0.7 Increased bilirubin 10 1.3 4.6 1.3 Graded according to NCI CTCAE v5.0.
Platinum-refractory Intolerant Locally Recurrent or Metastatic SCAC: ZYNYZ as a Single Agent
The safety of ZYNYZ in patients with platinum-refractory intolerant locally recurrent or metastatic SCAC was evaluated in 94 patients in the POD1UM‑202 trial [see Clinical Studies (14.1)]. Patients received ZYNYZ 500 mg intravenously every 4 weeks until disease progression, unacceptable toxicity, or up to 24 months. The median duration of exposure was 2.8 months (range: 1 day to 27.1 months).
Serious adverse reactions occurred in 40% of patients receiving ZYNYZ. The most frequent serious adverse reactions (≥ 2% of patients) were non-urinary tract infection, perineal pain, abdominal pain, anemia, hemorrhage, diarrhea, pyrexia, urinary tract infection, musculoskeletal pain, and dyspnea.
Permanent discontinuation of ZYNYZ due to an adverse reaction occurred in 4.3% of patients. These adverse reactions included diarrhea, non-urinary tract infection, perineal pain, and rash.
Dosage interruptions due to an adverse reaction occurred in 21% of patients who received ZYNYZ. Adverse reactions that resulted in dose delay in ≥ 2% of patients who received ZYNYZ were non-urinary tract infection, rash, diarrhea, abdominal pain, hemorrhage, musculoskeletal pain, pyrexia, and urinary tract infection.
The most common (≥ 10%) adverse reactions that occurred in patients receiving ZYNYZ were fatigue, musculoskeletal pain, diarrhea, non-urinary tract infections, perineal pain, hemorrhage, urinary tract infection, rash, nausea, decreased appetite, constipation, abdominal pain, dyspnea, pyrexia, vomiting, cough, pruritus, hypothyroidism, headache, and decreased weight.
Table 5 and Table 6 summarize adverse reactions and laboratory abnormalities, respectively, that occurred in POD1UM-202.
Table 5: Adverse Reactions in ≥ 10% of Patients with Platinum-Refractory Locally Recurrent or Metastatic SCAC Receiving ZYNYZ in POD1UM-202 - *
- Includes fatigue and asthenia.
- †
- Includes arthralgia, back pain, bone pain, musculoskeletal chest pain, myalgia, non-cardiac chest pain, osteoarthritis, pain in extremity, and spinal pain.
- ‡
- Includes diarrhea, gastroenteritis, and immune-mediated enterocolitis.
- §
- Includes abdominal pain, abdominal discomfort, and abdominal pain upper.
- ¶
- Includes anal abscess, cellulitis, cholangitis, cholecystitis, cholecystitis acute, device related infection, herpes zoster, Lyme disease, pelvic infection, peritonitis, Pneumocystis jirovecii pneumonia, pneumonia, postoperative wound infection, pseudomonas infection, sepsis, skin infection, stoma site infection, and wound infection bacterial.
- #
- Includes urinary tract infection, cystitis, escherichia urinary tract infection, and pyelonephritis.
- Þ
- Includes anorectal discomfort, pelvic pain, proctalgia, and vulvovaginal discomfort.
- ß
- Includes epistaxis, hematochezia, hematuria, proctitis hemorrhagic, rectal hemorrhage, stoma site hemorrhage, and vaginal hemorrhage.
- à
- Includes rash, dermatitis, dermatitis acneiform, eczema, erythema, palmar-plantar erythrodysesthesia syndrome, rash erythematous, and rash maculo-papular.
- è
- Includes decreased appetite and hypophagia.
- ð
- Includes cough and productive cough.
Adverse Reaction ZYNYZ
(N = 94)All Grades (%) Grades 3-4 (%) General disorders and administration site conditions Fatigue* 42 7 Pyrexia 14 2.1 Musculoskeletal and connective tissue disorders Musculoskeletal pain† 27 2.1 Gastrointestinal disorders Diarrhea‡ 23 2.1 Nausea 16 0 Constipation 15 0 Abdominal pain§ 14 3.2 Vomiting 14 1.1 Infections and infestations Non-urinary tract infections¶ 21 12 Urinary tract infection# 17 2.1 Reproductive system and breast disorders Perineal painÞ 19 7 Vascular disorders Hemorrhageß 19 3.2 Skin and subcutaneous tissue disorders Rashà 16 2.1 Pruritus 12 0 Metabolism and nutrition disorders Decreased appetiteè 15 2.1 Respiratory, thoracic, and mediastinal disorders Dyspnea 14 3.2 Coughð 13 0 Endocrine disorders Hypothyroidism 10 0 Nervous system disorders Headache 10 0 Investigations Decreased weight 10 0 Graded according to NCI CTCAE v5.0.
Table 6: Laboratory Abnormalities that Worsened from Baseline to Grade 3 or 4 Occurring in ≥ 1% of Patients with Platinum-Refractory Locally Recurrent or Metastatic SCAC Receiving ZYNYZ in POD1UM-202 - *
- The denominator used to calculate the rate varied from 59 to 87 based on the number of patients with a baseline value and at least one post-treatment value.
Laboratory abnormality ZYNYZ* All Grades (%) Grades 3-4 (%) Hematology Decreased hemoglobin 35 2.3 Decreased lymphocytes 33 6 Chemistry Decreased albumin 36 1.2 Increased aspartate aminotransferase 28 1.1 Decreased sodium 24 1.2 Increased triglycerides 19 3.4 Increased lipase 17 1.3 Increased alanine aminotransferase 16 1.1 Increased bilirubin 8 2.3 Graded according to NCI CTCAE v5.0. Merkel Cell Carcinoma (MCC)
Metastatic or Recurrent Locally Advanced MCC: ZYNYZ as a Single Agent
The safety of ZYNYZ was evaluated in 105 patients enrolled in the POD1UM-201 trial with metastatic or recurrent locally advanced MCC [see Clinical Studies (14.2)]. Patients received ZYNYZ 500 mg intravenously every 4 weeks until disease progression, unacceptable toxicity, or up to 24 months. The median duration of exposure was 5.6 months (range: 1 day to 23 months).
Serious adverse reactions occurred in 22% of patients receiving ZYNYZ. The most frequent serious adverse reactions (≥ 2% of patients) were fatigue, arrhythmia, and pneumonitis.
Permanent discontinuation of ZYNYZ due to an adverse reaction occurred in 11% of patients. These included asthenia, atrial fibrillation, concomitant disease progression of chronic lymphocytic leukemia, demyelinating polyneuropathy, eosinophilic fasciitis, increased transaminases, infusion-related reaction, lung disorder, pancreatitis, polyarthritis, and radiculopathy (1 patient each).
Dosage interruptions due to an adverse reaction occurred in 25% of patients who received ZYNYZ. Adverse reactions or laboratory abnormalities that required dosage interruption in ≥ 2% of patients who received ZYNYZ were increased transaminases, increased lipase, increased amylase, pneumonitis, and pyrexia.
The most common (≥ 10%) adverse reactions that occurred in patients receiving ZYNYZ were fatigue, musculoskeletal pain, pruritus, diarrhea, rash, pyrexia, and nausea.
Table 7 and Table 8 summarize adverse reactions and laboratory abnormalities, respectively, that occurred in POD1UM-201.
Table 7: Adverse Reactions in ≥ 10% of Patients with Metastatic or Recurrent Locally Advanced MCC Receiving ZYNYZ in POD1UM-201 Graded according to NCI CTCAE v5.0. Adverse Reaction
ZYNYZ
(N = 105)All Grades (%) Grades 3-4 (%) General disorders and administration site conditions Fatigue* 28 1 Pyrexia 10 0 Musculoskeletal and connective tissue disorders
Musculoskeletal pain† 22 2.9 Skin and subcutaneous tissue disorders Pruritus 18 0 Rash‡ 11 1 Gastrointestinal disorders Diarrhea 15 0 Nausea 10 0 Table 8: Laboratory Abnormalities that Worsened from Baseline to Grade 3 or 4 Occurring in ≥ 1% of Patients with Metastatic or Recurrent Locally Advanced MCC Receiving ZYNYZ in POD1UM-201 Graded according to NCI CTCAE v5.0. - *
-
The denominator used to calculate the rate varied from 86 to 92 based on the number of patients with a baseline value and at least one post-treatment value.
Laboratory Abnormality ZYNYZ*
All Grades (%)* Grades 3-4 (%)* Hematology
Decreased hemoglobin
38 1.1 Decreased lymphocytes
29 10 Decreased neutrophils 13 3.3 Decreased leukocytes
12 1.1 Chemistry Increased lipase
30 3.4 Decreased sodium
23 3.3 Increased aspartate aminotransferase
23 2.2 Increased alanine aminotransferase 21 3.3 Increased alkaline phosphatase 20 1.1 Increased amylase 19 1.2 Decreased potassium
9 1.1 Increased calcium
8 1.1 -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, ZYNYZ can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data ...
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, ZYNYZ can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of ZYNYZ in pregnant women. Animal studies have demonstrated that inhibition of the PD‑1/PD‑L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death (see Data). Human IgG4 immunoglobulins (IgG4) are known to cross the placenta; therefore, retifanlimab-dlwr has the potential to be transmitted from the mother to the developing fetus. Advise women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with ZYNYZ to evaluate its effect on reproduction and fetal development. A central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. In murine models of pregnancy, blockade of PD-L1 signaling has been shown to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering ZYNYZ during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1/PD-L1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to retifanlimab-dlwr may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There is no information regarding the presence of retifanlimab-dlwr in human milk, or its effects on the breastfed child or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to ZYNYZ are unknown. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 4 months after the last dose of ZYNYZ.
8.3 Females and Males of Reproductive Potential
ZYNYZ can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating ZYNYZ [see Use in Specific Populations (8.1)].
Contraception
Advise females of reproductive potential to use effective contraception during treatment with ZYNYZ and for 4 months after the last dose.
8.4 Pediatric Use
The safety and effectiveness of ZYNYZ have not been established in pediatric patients.
Close8.5 Geriatric Use
In Combination with Carboplatin and Paclitaxel
Of the 154 patients with inoperable locally recurrent or metastatic SCAC treated with ZYNYZ in combination with carboplatin and paclitaxel in POD1UM-303, 38% were 65 years or older, and 9% were 75 years or older [see Clinical Studies (Section 14.1)]. No overall differences in safety or effectiveness were observed between these patients and younger patients.
As a Single Agent
Of the 94 patients with locally recurrent or with metastatic SCAC treated with ZYNYZ in POD1UM‑202, 49% were 65 years or older, and 11% were 75 years or older [see Clinical Studies (14.1)].
Of the 65 patients with metastatic or recurrent locally advanced MCC treated with ZYNYZ in POD1UM-201, 79% were 65 years or older, and 37% were 75 years or older [see Clinical Studies (14.2)].
Clinical studies of ZYNYZ as a single agent did not include sufficient numbers of younger adult patients to determine if patients 65 years of age and older respond differently than younger adult patients.
-
11 DESCRIPTIONRetifanlimab-dlwr is a programmed death receptor-1 (PD-1)–blocking antibody. Retifanlimab‑dlwr is a humanized IgG4 kappa monoclonal antibody produced in Chinese hamster ovary cells ...
Retifanlimab-dlwr is a programmed death receptor-1 (PD-1)–blocking antibody. Retifanlimab‑dlwr is a humanized IgG4 kappa monoclonal antibody produced in Chinese hamster ovary cells. Retifanlimab-dlwr has an approximate molecular weight of 148 kDa.
ZYNYZ (retifanlimab-dlwr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale yellow solution for intravenous use. The solution is free from visible particles.
Each single-dose vial contains 500 mg of retifanlimab-dlwr in 20 mL of solution. Each mL contains 25 mg of retifanlimab-dlwr, glacial acetic acid (0.18 mg), polysorbate 80 (0.1 mg), sodium acetate (0.57 mg), sucrose (90 mg), and Water for Injection, USP. The pH is 5.1.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands ...
12.1 Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors.
Retifanlimab-dlwr binds to the PD-1 receptor, blocks interaction with its ligands PD-L1 and PD‑L2, and potentiates T-cell activity.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for safety and effectiveness of retifanlimab-dlwr have not been fully characterized.
12.3 Pharmacokinetics
The pharmacokinetics of retifanlimab-dlwr were evaluated in patients with various solid tumors, including patients with SCAC and patients with MCC. Retifanlimab-dlwr exposures (maximum concentration [Cmax] and area under the curve [AUC]) increased proportionally over a dosage range from 375 mg to 750 mg (0.75- to 1.5-fold of the approved recommended dose).
Following administration of retifanlimab-dlwr at 500 mg every 4 weeks, steady-state concentrations were achieved at Cycle 6 (approximately 6 months) and systemic accumulation was 1.3-fold.
Distribution
The geometric mean volume of distribution at steady state for retifanlimab-dlwr is 6.0 L (coefficient of variation [CV]: 20%).
Elimination
The elimination half-life of retifanlimab-dlwr at steady state is 19 days (CV: 30%). Clearance of retifanlimab-dlwr after the first dose was 0.3 L/day (CV: 38%) and decreased over time by approximately 23%, resulting in a steady-state clearance of 0.23 L/day.
Specific Populations
The following factors have no clinically meaningful effect on the pharmacokinetics of retifanlimab-dlwr: age (18 to 94 years), sex, body weight (33 to 133 kg), race (White, Black, Asian), albumin level (17 to 54 g/L), Eastern Cooperative Oncology Group (ECOG) score (0 to 2), tumor burden (sum of the target lesion diameters: 0 to 360 mm), HIV status, renal function (estimated glomerular filtration rate ≥ 26 mL/min/1.73 m2), or mild hepatic impairment (total bilirubin less than or equal to the ULN and AST greater than ULN or total bilirubin greater than ULN and less than or equal to 1.5 times ULN and any AST). The pharmacokinetics of retifanlimab-dlwr have not been studied in patients with moderate or severe hepatic impairment.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADAs in the studies described below with the incidence of ADAs in other studies, including those of ZYNYZ or of other retifanlimab products.
ADAs were tested in 153 patients with SCAC who received ZYNYZ in POD1UM-303 and 94 patients with SCAC who received ZYNYZ in POD1UM-202. The incidence of retifanlimab treatment-emergent ADAs was determined using a bridging enzyme-linked immunosorbent assay following a median exposure time of 226 days in POD1UM-303 and 85 days in POD1UM‑202. None of the patients in POD1UM‑303 or POD1UM-202 tested positive for retifanlimab treatment-emergent ADAs.
ADAs were tested in 104 patients with MCC who received ZYNYZ in POD1UM-201. The incidence of retifanlimab treatment-emergent ADAs was 2.9% (3/104) using a bridging enzyme‑linked immunosorbent assay following a median exposure time of 169 days. Neutralizing antibodies were detected in 2 of 3 patients with treatment-emergent ADAs. The effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of retifanlimab products is unknown.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed to assess the potential of retifanlimab-dlwr for carcinogenicity or genotoxicity. In 1-month and 3-month ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of retifanlimab-dlwr for carcinogenicity or genotoxicity.
In 1-month and 3-month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs; however, most animals in these studies were not sexually mature.
Close13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis–infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD-1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-L1 and PD-1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
-
14 CLINICAL STUDIES14.1 Squamous Cell Carcinoma of the Anal Canal (SCAC) ZYNYZ in Combination with Carboplatin and Paclitaxel for the Treatment of Inoperable Locally Recurrent or Metastatic SCAC - The efficacy ...
14.1 Squamous Cell Carcinoma of the Anal Canal (SCAC)
ZYNYZ in Combination with Carboplatin and Paclitaxel for the Treatment of Inoperable Locally Recurrent or Metastatic SCAC
The efficacy of ZYNYZ in combination with carboplatin and paclitaxel was evaluated in study POD1UM-303/InterAACT 2 (NCT04472429), a randomized, multicenter, double-blind study in 308 patients with chemotherapy-naïve inoperable locally recurrent or metastatic SCAC. Patients with active autoimmune disease or a medical condition that required immunosuppression, severe hepatic or renal impairment, clinically significant cardiac disease, history of organ transplant, or Eastern Cooperative Oncology Group (ECOG) performance score (PS) ≥ 2 were ineligible. Patients who had not received prior systemic therapy other than a radiosensitizing agent or neoadjuvant or adjuvant therapy completed > 6 months prior to study entry were eligible, as were patients who were HIV-positive if they had an undetectable viral load, a CD4+ count ≥ 200 cells/microliter, and were receiving antiretroviral therapy. The study did not enroll patients who received prior treatment with PD-(L)1‑directed therapies.
Patients were randomized (1:1) to receive either:
- ZYNYZ 500 mg intravenously every 4 weeks on Day 1, carboplatin AUC of 5 on Day 1, and paclitaxel 80 mg/m2 on Days 1, 8, and 15 for 6 cycles followed by ZYNYZ 500 mg intravenously every 4 weeks, or
- Placebo intravenously every 4 weeks on Day 1, carboplatin AUC 5 mg/mL on Day 1, and paclitaxel 80 mg/m2 on Days 1, 8, and 15 for 6 cycles followed by placebo 500 mg intravenously every 4 weeks.
Randomization was stratified by PD-L1 expression (< 1% versus ≥ 1%), region, and extent of disease (locally recurrent versus metastatic). Treatment with ZYNYZ continued until disease progression, unacceptable toxicity, death, or withdrawal of consent, for up to 12 months. Tumor response assessments were performed every 8 weeks throughout the treatment period. Patients randomized to the placebo arm were offered ZYNYZ as a single agent at the time of disease progression.
The major efficacy outcomes were progression-free survival (PFS) as assessed by a blinded independent central review (BICR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and overall survival (OS). Additional efficacy outcome measures included objective response rate (ORR) and duration of response (DOR), as assessed by BICR.
Among the 308 patients evaluated, the median age was 62 years (range: 29-86); 72% female, 87% White, 1.6% Black, 6% Asian, 3.2% race unknown or not reported; 7% were Hispanic or Latino, 85% were not Hispanic or Latino, 8% ethnicity unknown or not reported; 55% had an ECOG performance status (PS) of 0 and 45% had an ECOG PS of 1; 3.6% were HIV-positive and 96% had unknown or negative HIV status. Thirty-five percent of patients had prior surgery, and 71% of patients had prior radiotherapy. Eighty-three percent of patients had metastatic disease at baseline. PD-L1 expression of ≥ 1% was present in 91% of tumors. Of the 199 patients with tumor tissue available for central review, 150 (75%) were positive for human papillomavirus P16 protein expression.
Efficacy results of the final analysis of PFS and interim analysis of OS are summarized in Table 9 and Figure 1. There were 69 patients (45%) from the placebo arm who received ZYNYZ as a single agent after disease progression. OS results were not statistically significant at this interim analysis.
Table 9: Efficacy Results from Study POD1UM-303/InterAACT 2 Endpoint ZYNYZ in Combination with Carboplatin and Paclitaxel
(N = 154)Placebo in Combination with Carboplatin and Paclitaxel
(N = 154)Progression-free survival Events, n (%)
92 (60) 110 (71) Median (months) (95% CI)
9.3 (7.5, 11.3) 7.4 (7.1, 7.7) Hazard ratio* (95% CI)
0.63 (0.47, 0.84) p-value†
0.0006 Overall survival Deaths, n (%) 53 (34) 73 (47) Median (months) (95% CI) 29.2 (24.2, NE) 23 (15.1, 27.9) Hazard ratio* (95% CI) 0.70 (0.49, 1.01) Objective response rate Objective response rate (%) (95% CI) 56 (48, 64) 44 (36, 52) Complete response, n (%)
34 (22) 21 (14) Partial response, n (%)
52 (34) 47 (31) Median duration of response, in months (95% CI) 14.0 (8.6, 22.2) 7.2 (5.6, 9.3) CI = confidence interval; NE = not evaluable. Figure 1: Kaplan-Meier Curves for PFS in POD1UM-303/InterAACT 2
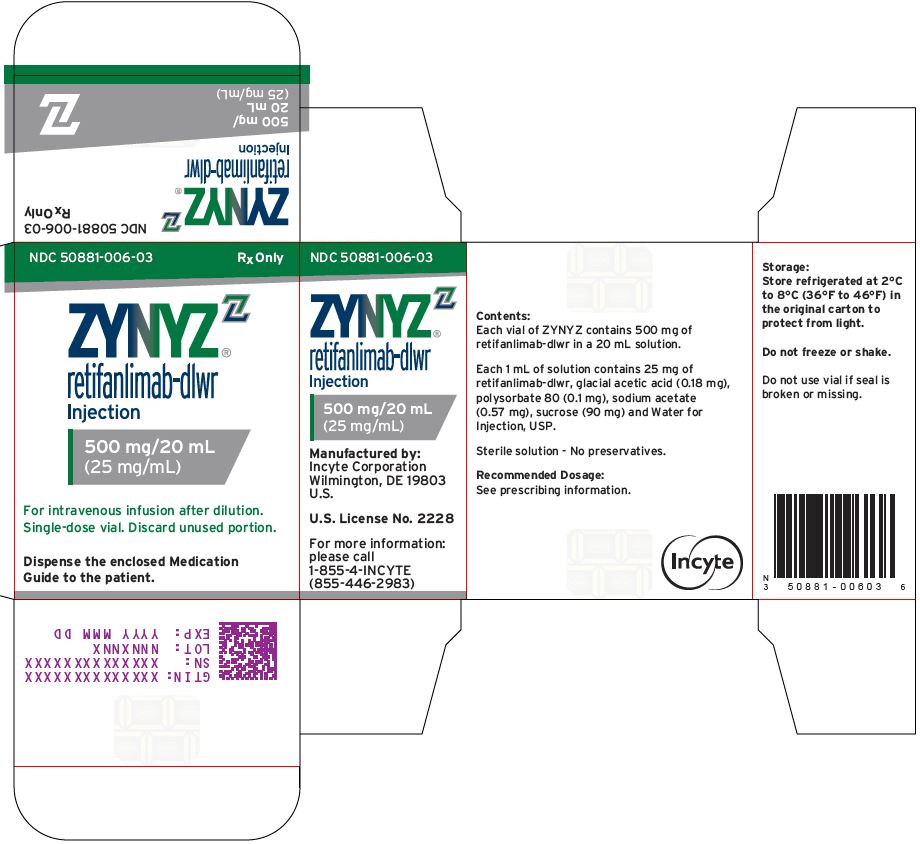
Figure 1: Kaplan-Meier Curves for PFS in POD1UM-303/InterAACT 2
Study POD1UM-202: As a Single Agent for the Treatment of Platinum-refractory or Intolerant Locally Recurrent or Metastatic Squamous Cell Carcinoma of the Anal Canal
The efficacy of ZYNYZ was evaluated in study POD1UM-202 (NCT03597295), an open-label, multicenter, single-arm study in 94 patients with locally recurrent or metastatic SCAC who progressed on or were intolerant of platinum-based chemotherapy. Patients with active autoimmune disease or a medical condition that required immunosuppression or ECOG PS ≥ 2 were ineligible. Patients who were HIV-positive, had an undetectable viral load, a CD4+ count ≥ 300 cells/microliter, and were receiving antiretroviral therapy were eligible.
Patients received ZYNYZ 500 mg intravenously every 4 weeks until disease progression, unacceptable toxicity, or up to 24 months. Tumor response assessments were performed every 8 weeks throughout the treatment period.
The major efficacy outcomes were ORR and DOR as assessed by an independent central review committee (IRC) according to RECIST v1.1.
The median age of enrolled patients was 64 years (range: 37-94); 49% ≥ 65 years; 65% female, 77% White, 1.1% Black, 22% race unknown or not reported; 4.3% were Hispanic or Latino, 52% were not Hispanic or Latino, 44% ethnicity unknown or not reported; 42% had an ECOG PS of 0 and 59% had an ECOG PS of 1; 10% were HIV‑positive. Forty-six percent of patients had prior surgery and 87% of patients had prior radiotherapy. Eighty-one percent of patients had metastatic disease at baseline. Of the 58 patients with tumor tissue available for review, 54 (93%) were positive for HPV.
Efficacy results from POD1UM‑202 are summarized in Table 10.
Table 10: Efficacy Results from Study POD1UM-202 Endpoint ZYNYZ
(N = 94)Objective Response Rate (95% CI) 14% (8, 23)
Complete response, n (%) 1 (1.1)
Partial response, n (%) 12 (13) Duration of Response Median, months
9.5 (4.4, NE) Patients with DOR ≥ 6 months (95% CI) 65% (31, 85) Patients with DOR ≥ 12 months (95% CI) 41% (11, 69) CI = confidence interval; DOR = duration of response; NE = not evaluable.
Close14.2 Merkel Cell Carcinoma (MCC)
The efficacy of ZYNYZ was evaluated in study POD1UM-201 (NCT03599713), an open-label, multiregional, single-arm study in 65 patients with metastatic or recurrent locally advanced MCC who had not received prior systemic therapy for their advanced disease. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible. Patients who were HIV-positive, with an undetectable viral load, a CD4+ count ≥ 300 cells/microliter and receiving antiretroviral therapy were eligible.
Patients received ZYNYZ 500 mg intravenously every 4 weeks until disease progression, unacceptable toxicity, or up to 24 months. Tumor response assessments were performed every 8 weeks for the first year of therapy and 12 weeks thereafter.
The major efficacy outcomes were ORR and DOR as assessed by an IRC according to RECIST v1.1.
The median age of enrolled patients was 71 years (range: 44-90); 37% were ≥ 75 years; 65% of patients were male; 78% of patients were White, 20% were race unknown or not reported, 2% were Asian; 74% had an ECOG PS of 0 and 26% had an ECOG PS of 1; 98% were HIV-negative. Seventy-two percent of patients had prior surgery and 38% of patients had prior radiotherapy. Eighty-eight percent of patients had metastatic disease at baseline. Tumor samples were evaluated for Merkel cell polyomavirus (MCPyV): 71% were positive, 23% negative, 2% equivocal, and 5% missing.
Efficacy results are summarized in Table 11.
Table 11: Efficacy Results from Study POD1UM-201 CI = confidence interval; DOR = duration of response; + denotes ongoing response. Endpoint ZYNYZ
(N = 65)Objective Response Rate (95% CI) 52% (40, 65) Complete responses, n (%) 12 (18) Partial responses, n (%) 22 (34)
Duration of Response N = 34 Range, months 1.1 to 24.9+
Patients with DOR ≥ 6 months, n (%)
26 (76)
Patients with DOR ≥ 12 months, n (%)
21 (62)
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZYNYZ (retifanlimab-dlwr) injection is a clear to slightly opalescent, colorless to pale yellow solution. It is supplied in a carton containing one single-dose vial of: 500 mg/20 mL (25 mg/mL ...
ZYNYZ (retifanlimab-dlwr) injection is a clear to slightly opalescent, colorless to pale yellow solution. It is supplied in a carton containing one single-dose vial of:
- 500 mg/20 mL (25 mg/mL) (NDC 50881-006-03)
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or shake.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Immune-Mediated Adverse Reactions - Advise patients that ZYNYZ can cause immune-mediated adverse reactions ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Advise patients that ZYNYZ can cause immune-mediated adverse reactions, including the following [see Warnings and Precautions (5.1)]:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of pneumonitis, including new or worsening symptoms of cough, chest pain, or shortness of breath.
- Colitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of colitis, including diarrhea, blood or mucus in stools, or severe abdominal pain.
- Hepatitis: Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatitis.
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypothyroidism, hyperthyroidism, adrenal insufficiency, hypophysitis, or type 1 diabetes mellitus.
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis.
- Dermatologic Adverse Reactions: Advise patients to contact their healthcare provider immediately if they develop a new rash.
- Other immune-mediated adverse reactions:
- Advise patients that immune-mediated adverse reactions can occur and may involve any organ system, and to contact their healthcare provider immediately for any new or worsening signs or symptoms [see Warnings and Precautions (5.1)].
- Advise patients of the risk of solid organ transplant rejection and other transplant (including corneal graft) rejection [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Complications of Allogeneic HSCT or Solid Organ Transplant Rejection
Advise patients to contact their healthcare provider immediately if they develop signs or symptoms of post-allogeneic HSCT complications [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise females of reproductive potential that ZYNYZ can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of ZYNYZ [see Use in Specific Populations (8.3)].
Lactation
Advise female patients not to breastfeed while taking ZYNYZ and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Manufactured by:
Incyte Corporation
Wilmington, DE 19803
U.S. License No. 2228ZYNYZ and the ZYNYZ logo are registered trademarks of Incyte.
Close
Patent Information: www.incyte.com/patents
© 2025 Incyte Corporation. All rights reserved. -
MEDICATION GUIDEMEDICATION GUIDE - ZYNYZ® (ZYE-niz) (retifanlimab-dlwr) injection - What is the most important information I should know about ZYNYZ? ZYNYZ is a ...
MEDICATION GUIDE
ZYNYZ® (ZYE-niz)
(retifanlimab-dlwr)
injectionWhat is the most important information I should know about ZYNYZ?
ZYNYZ is a medicine that may treat certain types of cancers by working with your immune system. ZYNYZ can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including:
Lung problems. - cough
- shortness of breath
- chest pain
Intestinal problems. - diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems. - yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems. - headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems. - decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems. - rash
- itching
- skin blistering or peeling
- painful sores or ulcers in your mouth or nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with ZYNYZ. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
- chest pain, irregular heartbeat, shortness of breath, or swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- persistent or severe muscle pain or weakness, muscle cramps
- low red blood cells, bruising
Infusion reactions that can sometimes be severe. Signs and symptoms of infusion reactions may include:
- chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feel like passing out
- fever
- back or neck pain
Rejection of a transplanted organ or tissue. Your healthcare provider should tell you what signs and symptoms you should report and monitor you, depending on the type of organ or tissue transplant that you have had.
Complications, including graft-versus-host disease, in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with ZYNYZ. Your healthcare provider will monitor you for these complications.
Getting medical treatment right away may help keep these problems from becoming more serious.Your healthcare provider will check you for these problems during your treatment with ZYNYZ. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with ZYNYZ if you have severe side effects.
What is ZYNYZ?
ZYNYZ is a prescription medicine used to treat adults with:
- a type of anal cancer called squamous cell carcinoma of the anal canal (SCAC).
- ZYNYZ may be used in combination with the chemotherapy medicines carboplatin and paclitaxel, as your first treatment, when your anal cancer:
- has returned and cannot be removed by surgery, or
- has spread.
- ZYNYZ may be used alone when your anal cancer:
- has returned or has spread, and
- you have received chemotherapy that contains platinum, and it did not work, is no longer working, or you could not tolerate it.
- ZYNYZ may be used in combination with the chemotherapy medicines carboplatin and paclitaxel, as your first treatment, when your anal cancer:
- a type of skin cancer called Merkel cell carcinoma (MCC). ZYNYZ may be used alone to treat your skin cancer when it has spread or returned.
It is not known if ZYNYZ is safe and effective in children.
Before you receive ZYNYZ, tell your healthcare provider about all of your medical conditions, including if you:
- have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus
- have received an organ or tissue transplant, including corneal transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have received radiation treatment to your chest area
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. ZYNYZ can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with ZYNYZ.
- You should use an effective method of birth control during your treatment and for 4 months after your last dose of ZYNYZ. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ZYNYZ.
- are breastfeeding or plan to breastfeed. It is not known if ZYNYZ passes into your breast milk. Do not breastfeed during treatment and for 4 months after your last dose of ZYNYZ.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive ZYNYZ?
- Your healthcare provider will give you ZYNYZ into your vein through an intravenous (IV) line over 30 minutes.
- ZYNYZ is usually given every 4 weeks.
- Your healthcare provider will decide how many treatments you will need.
- Your healthcare provider will do blood tests to check you for side effects.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of ZYNYZ?
ZYNYZ can cause serious side effects. See "What is the most important information I should know about ZYNYZ?"
The most common side effects of ZYNYZ when given with the chemotherapy medicines carboplatin and paclitaxel in people with SCAC include:
- tiredness
- numbness, pain, tingling or burning in your hands or feet
- nausea
- hair loss
- diarrhea
- muscle and bone pain
- constipation
- bleeding
- rash
- vomiting
- decreased appetite
- itching
- stomach-area pain
The most common side effects of ZYNYZ when used alone in people with SCAC include: - tiredness
- muscle and bone pain
- diarrhea
- infection
- rectal or genital-area pain
- bleeding
- urinary tract infection (UTI)
- rash
- nausea
- loss of appetite
- constipation
- stomach-area pain
- shortness of breath
- fever
- vomiting
- cough
- itching
- low levels of thyroid hormone
- headache
- decreased weight
The most common side effects of ZYNYZ when used alone in people with MCC include: - tiredness
- muscle and bone pain
- itching
- diarrhea
- rash
- fever
- nausea
These are not all the possible side effects of ZYNYZ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ZYNYZ.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about ZYNYZ, talk with your healthcare provider. You can ask your healthcare provider for information about ZYNYZ that is written for health professionals.
What are the ingredients in ZYNYZ?
Active ingredient: retifanlimab-dlwr
Inactive ingredients: glacial acetic acid, polysorbate 80, sodium acetate, sucrose, and Water for Injection.
Manufactured by: Incyte Corporation, Wilmington, DE 19803
U.S. License No. 2228
ZYNYZ and the ZYNYZ logo are registered trademarks of Incyte.
© 2025 Incyte Corporation. All rights reserved.
Patent Information: www.incyte.com/patents
For more information, call 1-855-463-3463.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 5/2025
Close -
ZYNYZ 500 MG/20 ML CARTONNDC 50881-006-03 - Rx Only - ZYNYZ™ retifanlimab-dlwr - Injection - 500 mg/20 mL - (25 mg/mL) For intravenous infusion after dilution. Single-dose vial. Discard unused portion. Dispense the enclosed ...
NDC 50881-006-03
Rx OnlyZYNYZ™
retifanlimab-dlwr
Injection500 mg/20 mL
(25 mg/mL)For intravenous infusion after dilution.
Single-dose vial. Discard unused portion.Dispense the enclosed Medication
Close
Guide to the patient

-
INGREDIENTS AND APPEARANCEProduct Information
ZYNYZ retifanlimab-dlwr injection Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50881-006 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RETIFANLIMAB (UNII: 2Y3T5IF01Z) (RETIFANLIMAB - UNII:2Y3T5IF01Z) RETIFANLIMAB 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM ACETATE (UNII: 4550K0SC9B) .95 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) .18 mg in 1 mL SUCROSE (UNII: C151H8M554) 90 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) .1 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50881-006-03 1 in 1 CARTON 03/22/2023 1 20 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761334 03/22/2023
CloseLabeler - Incyte Corporation (556967347)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ZYNYZ- retifanlimab-dlwr injection
Number of versions: 4
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jun 2, 2025 | 10 (current) | download |
| Apr 18, 2024 | 7 | download |
| Nov 22, 2023 | 5 | download |
| Apr 4, 2023 | 4 | download |
RxNorm
ZYNYZ- retifanlimab-dlwr injection
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2632985 | retifanlimab-dlw 500 MG in 20 ML Injection | PSN |
| 2 | 2632985 | 20 ML retifanlimab-dlwr 25 MG/ML Injection | SCD |
| 3 | 2632985 | retifanlimab-dlw 500 MG per 20 ML Injection | SY |
| 4 | 2632991 | ZYNYZ 500 MG in 20 ML Injection | PSN |
| 5 | 2632991 | 20 ML retifanlimab-dlwr 25 MG/ML Injection [Zynyz] | SBD |
| 6 | 2632991 | 20 ML Zynyz 25 MG/ML Injection | SY |
| 7 | 2632991 | Zynyz 500 MG per 20 ML Injection | SY |
Get Label RSS Feed for this Drug
ZYNYZ- retifanlimab-dlwr injection
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=109648d0-d30a-42fc-8273-39cb1540a751
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ZYNYZ- retifanlimab-dlwr injection
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50881-006-03 |

