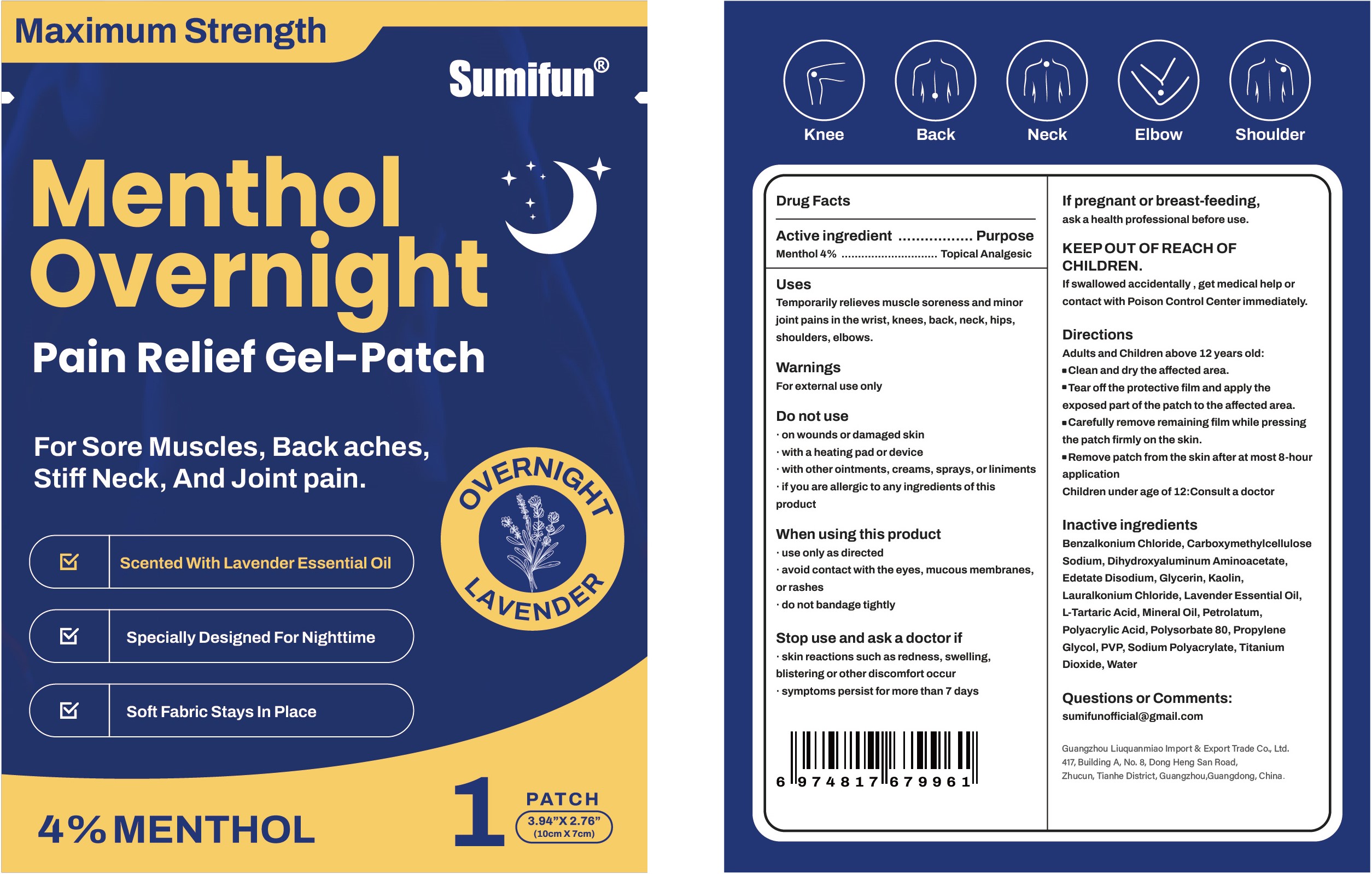
Label: MENTHOL OVERNIGHT PAIN RELIEF GEL-PATCH- menthol patch
- NDC Code(s): 83602-123-01, 83602-123-15
- Packager: Guangzhou Liuquanmiao Import & Export Trade Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- KEEP OUT OF REACH OF CHILDREN.
-
Directions
Adult and Children above 12 years old:
■ Clean and dry the affected area.
■ Tear off the protective film and apply the exposed part of the patch to the affected area.
■ Carefully remove remaining film while pressing the patch firmly on the skin.
■ Remove patch from the skin after at most 8-hour application.
Children under age of 12: Consult a doctor
-
Inactive ingredients
Benzalkonium Chloride, Carboxymethylcellulose Sodium, Dihydroxyaluminum Aminoacetate, Edetate Disodium, Glycerin, Kaolin, Lauralkonium Chloride, Lavender Essential Oil, L-Tartaric Acid, Mineral Oil, Petrolatum, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, PVP, Sodium Polyacrylate, Titanium Dioxide, Water
- Questions or Comments:
- Package label. Principal display panel
-
INGREDIENTS AND APPEARANCE
MENTHOL OVERNIGHT PAIN RELIEF GEL-PATCH
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83602-123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) WATER (UNII: 059QF0KO0R) LAURALKONIUM CHLORIDE (UNII: 07HUP5A29X) LAVENDER OIL (UNII: ZBP1YXW0H8) KAOLIN (UNII: 24H4NWX5CO) TARTARIC ACID (UNII: W4888I119H) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) PETROLATUM (UNII: 4T6H12BN9U) POVIDONE K90 (UNII: RDH86HJV5Z) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83602-123-15 15 in 1 BOX 02/05/2024 1 NDC:83602-123-01 5.5 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/05/2024 Labeler - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Registrant - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Establishment Name Address ID/FEI Business Operations Shanghai Chuangshi Medical Technology (Group) Co., Ltd. 546872672 manufacture(83602-123) , label(83602-123)