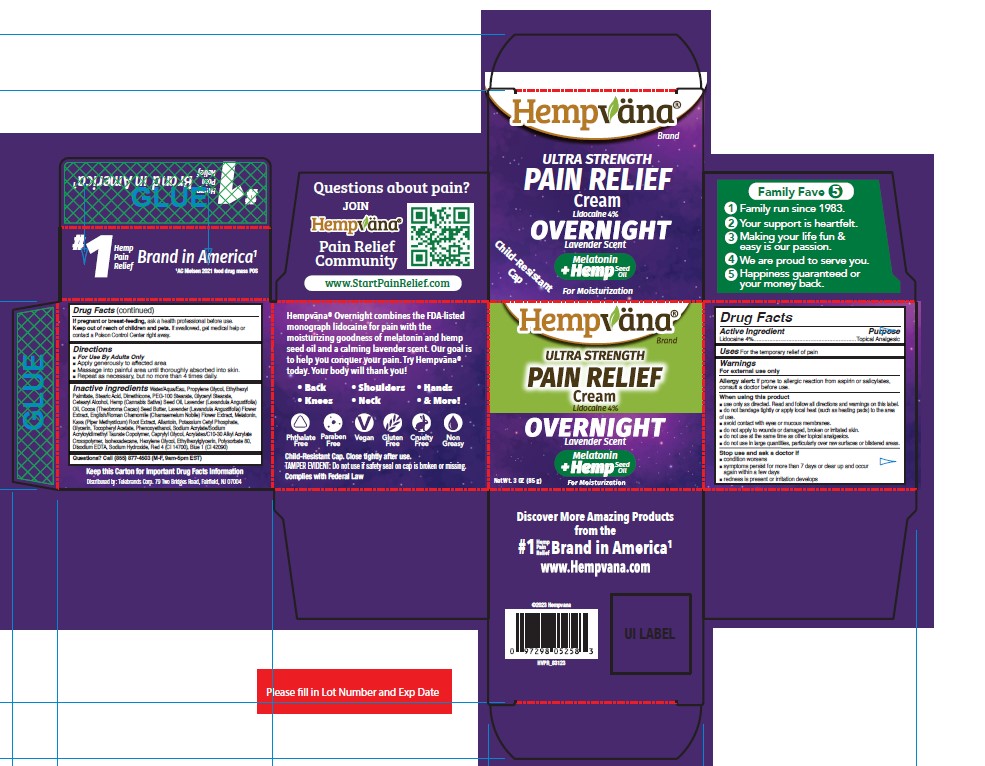
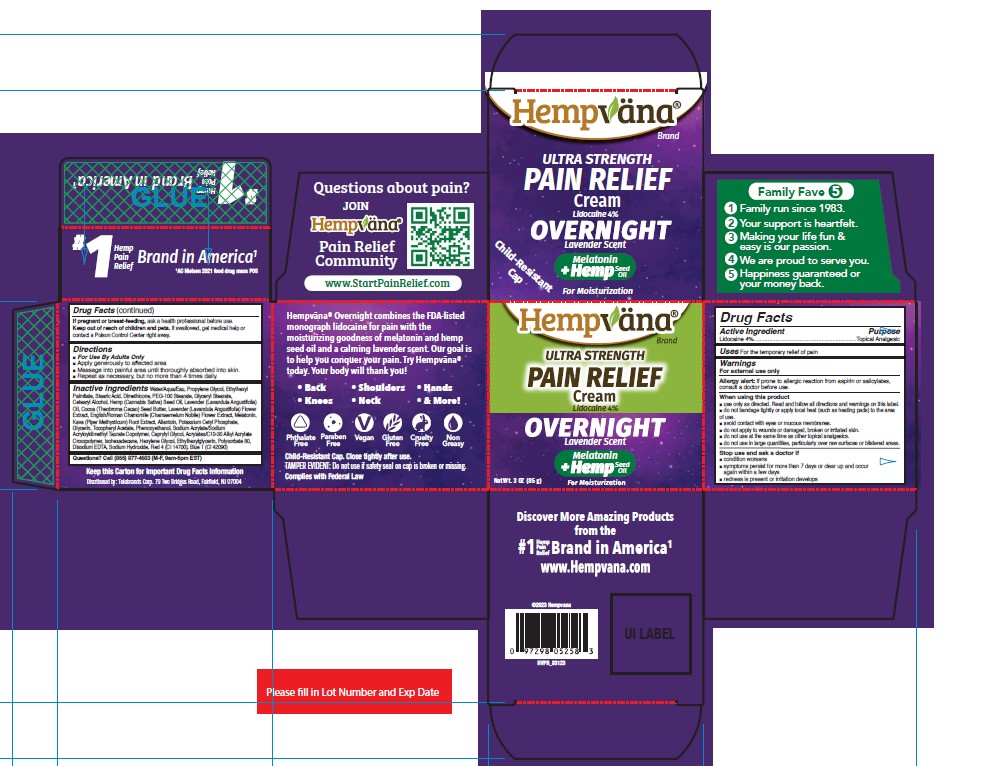
Label: HEMPVANA ULTRA STRENGTH PAIN RELIEF - OVERNIGHT- lidocaine cream
- NDC Code(s): 73287-029-01
- Packager: Telebrands Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
■ use only as directed. Read and follow all directions and warnings on this label.
■ do not bandage tightly or apply local heat (such as heating pads) to the area
of use.
■ avoid contact with eyes or mucous membranes.
■ do not apply to wounds or damaged, broken or irritated skin.
■ do not use at the same time as other topical analgesics.
■ do not use in large quantities, particularly over raw surfaces or blistered areas. - Directions
-
Inactive Ingredients
Water/Aqua/Eau, Propylene Glycol, Ethylhexyl Palmitate, Stearic Acid, Dimethicone, PEG-100 Stearate, Glyceryl Stearate, Cetearyl Alcohol, Hemp (Cannabis Sativa) Seed Oil, Lavender (Lavandula Angustifolia) Oil, Cocoa (Theobroma Cacao) Seed Butter, Lavender (Lavandula Angustifolia) Flower Extract, English/Roman Chamomile (Chamaemelum Nobile) Flower Extract, Melatonin, Kava (Piper Methysticum) Root Extract, Allantoin, Potassium Cetyl Phosphate, Glycerin, Tocopheryl Acetate, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Caprylyl Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Isohexadecane, Hexylene Glycol, Ethylhexylglycerin, Polysorbate 80, Disodium EDTA, Sodium Hydroxide, Red 4 (CI 14700), Blue 1 (CI 42090)
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMPVANA ULTRA STRENGTH PAIN RELIEF - OVERNIGHT
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73287-029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) SODIUM HYDROXIDE (UNII: 55X04QC32I) FD&C RED NO. 4 (UNII: X3W0AM1JLX) STEARIC ACID (UNII: 4ELV7Z65AP) LAVANDULA ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) LAVENDER OIL (UNII: ZBP1YXW0H8) ALLANTOIN (UNII: 344S277G0Z) PIPER METHYSTICUM ROOT (UNII: BOW48C81XP) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCOA BUTTER (UNII: 512OYT1CRR) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) MELATONIN (UNII: JL5DK93RCL) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73287-029-01 1 in 1 CARTON 01/30/2024 1 85 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/30/2024 Labeler - Telebrands Corp (177266558) Establishment Name Address ID/FEI Business Operations Neutraderm, Inc. 146224444 manufacture(73287-029)