Label: HEMORRHOID OINTMENTS- lidocaine paste
- NDC Code(s): 84023-401-01
- Packager: Shenzhen Yangan Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
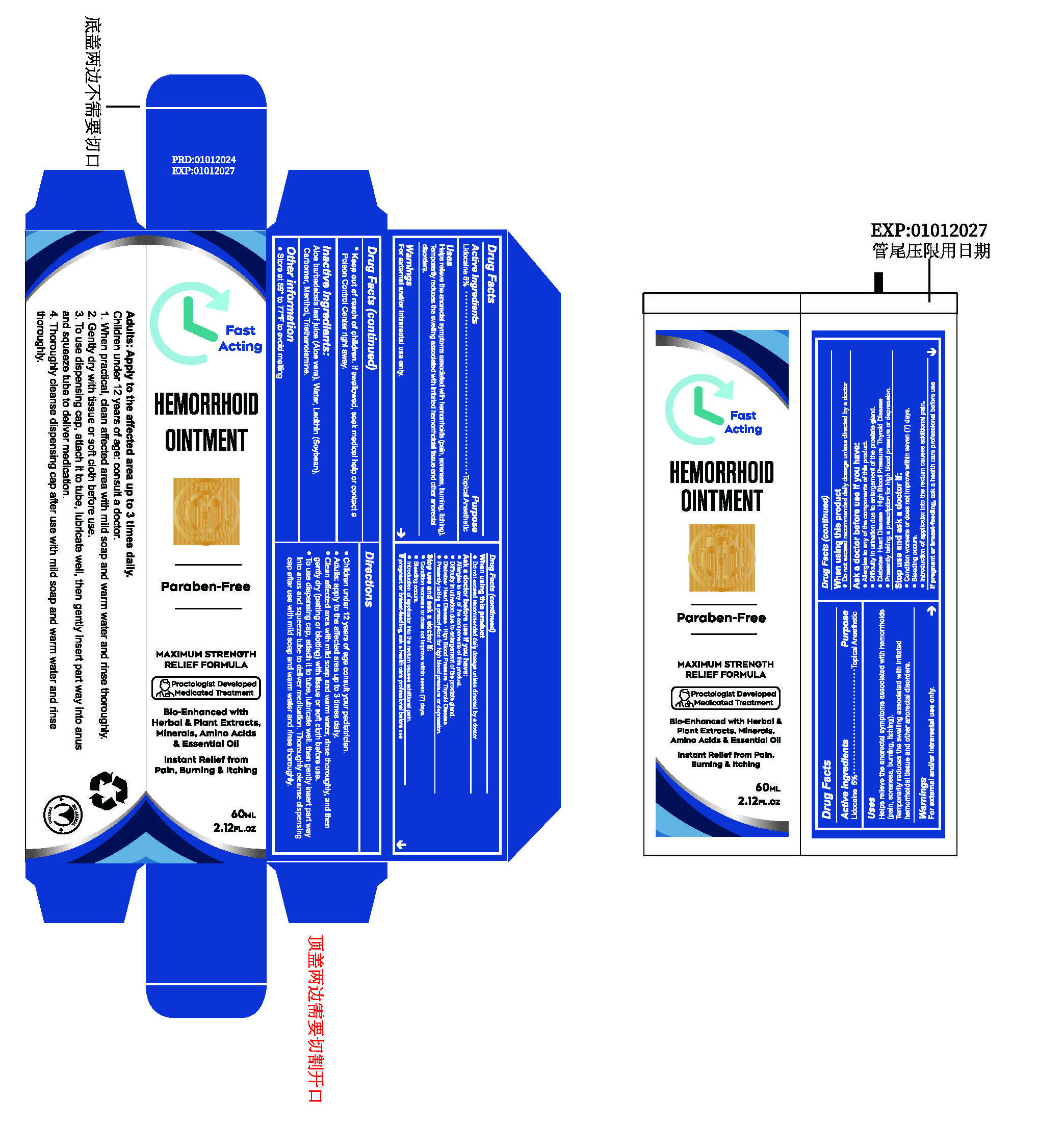
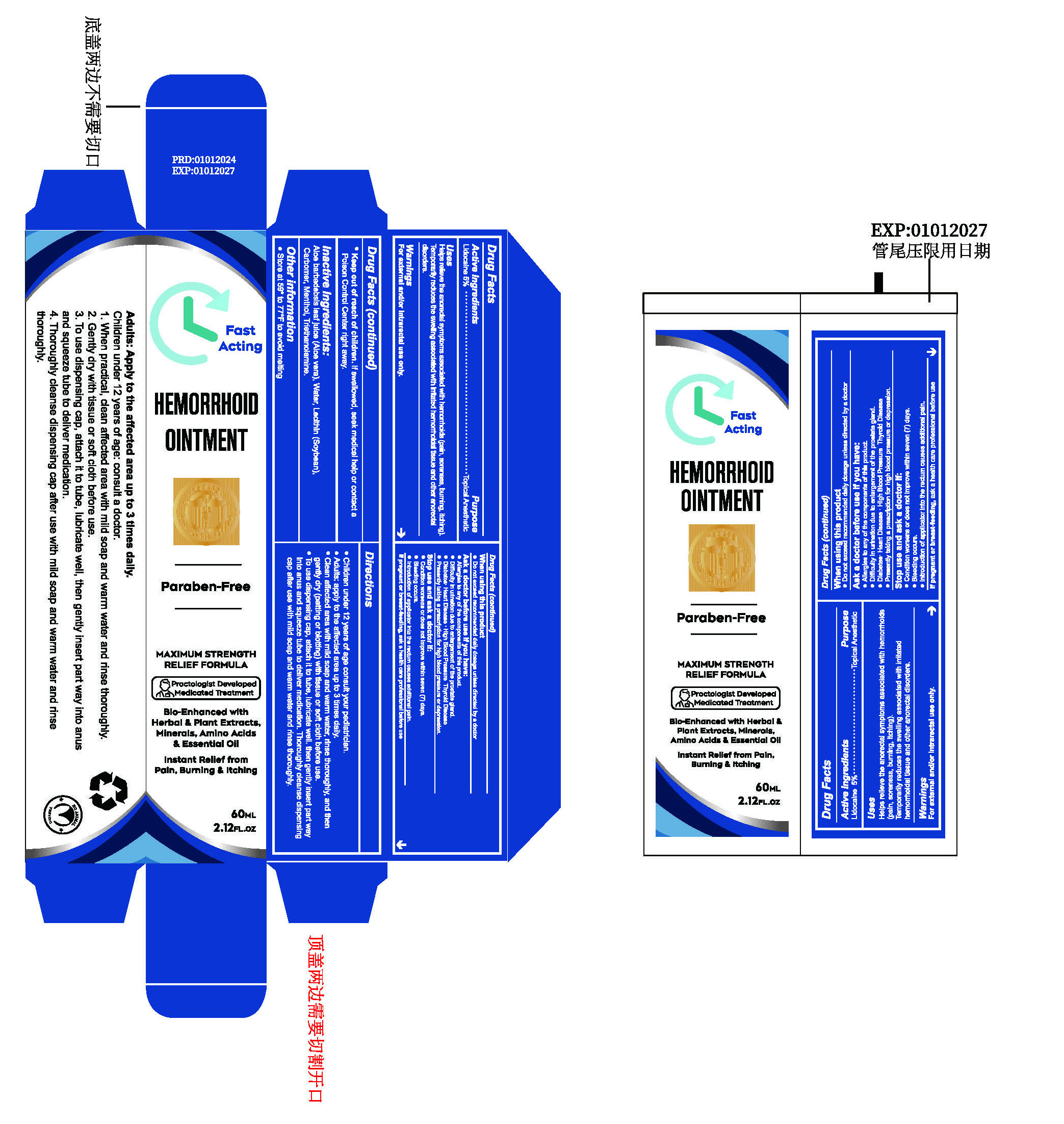
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
WHEN USING
Do not exceed recommended daily dosage unless directed by a doctor.
Ask a doctor before use if you have:
Allergies to any of the components of this product.
Difficulty in urination due to enlargement of the prostate gland.
Diabetes: Heart Disease : High Blood Pressure Thyroid Disease.
Presently taking a prescription for high blood pressure or depression. - STOP USE
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Children under 12 years of age consult your pediatrician.
Adults: apply to the affected area up to 3 times daily.
Clean affected area with mild soap and warm water, rinse thoroughly, and then gently dry (patting or blotting) with tissue or soft cloth before use.
To use dispensing cap, attach it to tube, lubricate well. then gently insert part way into anus and squeeze tube to deliver medication. Thoroughly cleanse dispensing cap after use with mild soap and warm water and rinse thoroughly. - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORRHOID OINTMENTS
lidocaine pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84023-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) MENTHOL (UNII: L7T10EIP3A) ALOE (UNII: V5VD430YW9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84023-401-01 1 in 1 BOX 01/30/2024 1 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/30/2024 Labeler - Shenzhen Yangan Technology Co., Ltd. (419283765) Establishment Name Address ID/FEI Business Operations Shenzhen Yangan Technology Co., Ltd. 419283765 manufacture(84023-401)