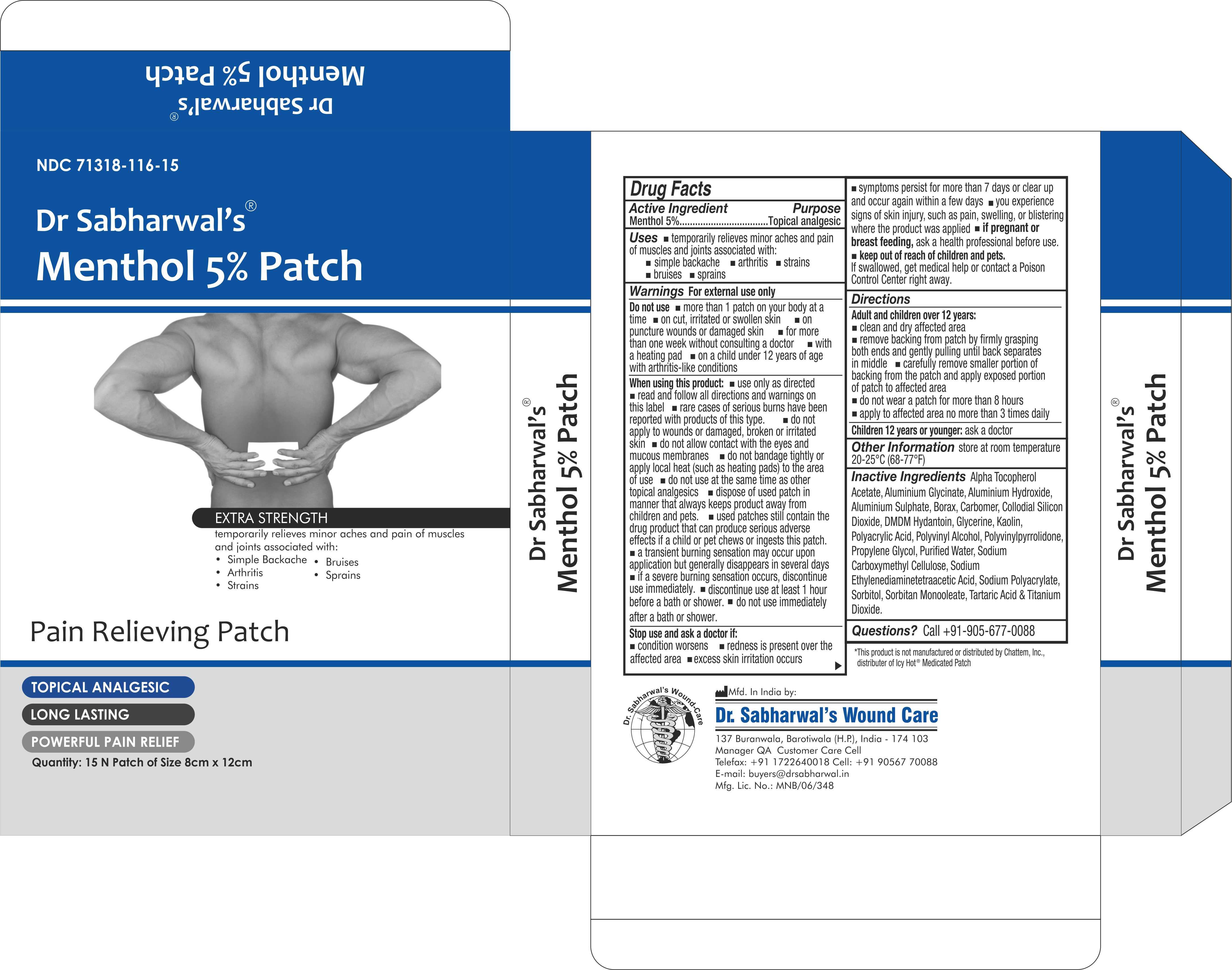
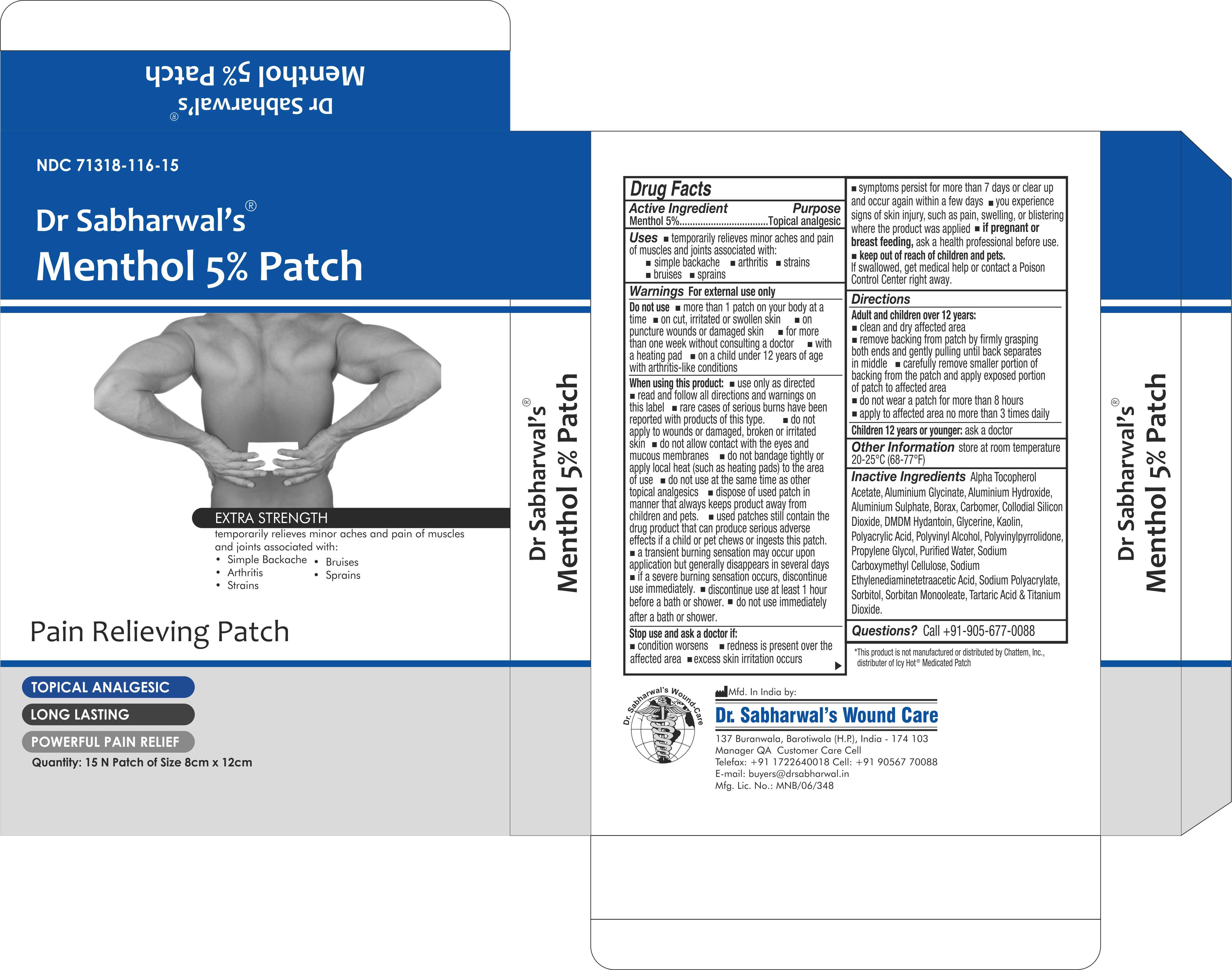
Label: DR SABHARWALS- menthol 5% patch patch
- NDC Code(s): 71318-116-15
- Packager: Dr. Sabharwal's Wound Care
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
WHEN USING
- use only as directed.
- read and follow all directions and warnings on this label
- rare cases of serious burns have been reported with products of this type.
- do not apply to wounds or damaged, broken or irritated skin
- do not allow contact with eyes and mucous membranes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not use at the same time as other topical analgesics
- dispose of used patch in manner that always keeps product away from children and pets
- used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch
- a transient burning sensation may occur upon application but generally disappears in several days
- if a severe burning sensation occurs, discontinue use immediately.
- discontinue use at least 1 hour before a bath or shower.
- do not use immediately after a bath or shower.
- ASK DOCTOR
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INSTRUCTIONS FOR USE
Adult and children over 12years:
- Clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until back separates in middle
- carefully remove smaller portion of backing from the patch and apply exposed portion of patch to affected area
- do not wear a patch for more than 8 hours
- apply affected area no more than 3 times daily
children 12 years or younger:
- ask a doctor
- STORAGE AND HANDLING
- QUESTIONS
-
INACTIVE INGREDIENT
Alpha Tocopherol Acetate, Aluminium Glycinate, Aluminium Hydroxide, Aluminium Sulphate, Borax, Carbomer, Collodial Silicon Dioxide, DMDM Hydantoin, Glycerine, Kaolin, Polyacrylic Acid, Polyvinyl Alcohol, Polyvinylpyrrolidone, Propylene Glycol, Purified Water, Sodium Carboxymethyl Cellulose, Sodium Ethylenediaminetetraacetic Acid, Sodium Polyacrylate, Sorbitol, Sorbitan Monooleate, Tartaric Acid & Titanium Dioxide.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR SABHARWALS
menthol 5% patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71318-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 240 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYVINYL ALCOHOL (100000 MW) (UNII: 949E52Z6MY) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TARTARIC ACID (UNII: W4888I119H) KAOLIN (UNII: 24H4NWX5CO) DMDM HYDANTOIN (UNII: BYR0546TOW) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER 940 (UNII: 4Q93RCW27E) Product Characteristics Color white Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71318-116-15 15 in 1 CARTON 01/30/2024 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/30/2024 Labeler - Dr. Sabharwal's Wound Care (862184668) Registrant - Dr. Sabharwal's Wound Care (862184668) Establishment Name Address ID/FEI Business Operations Dr. Sabharwal's Wound Care 862184668 manufacture(71318-116)