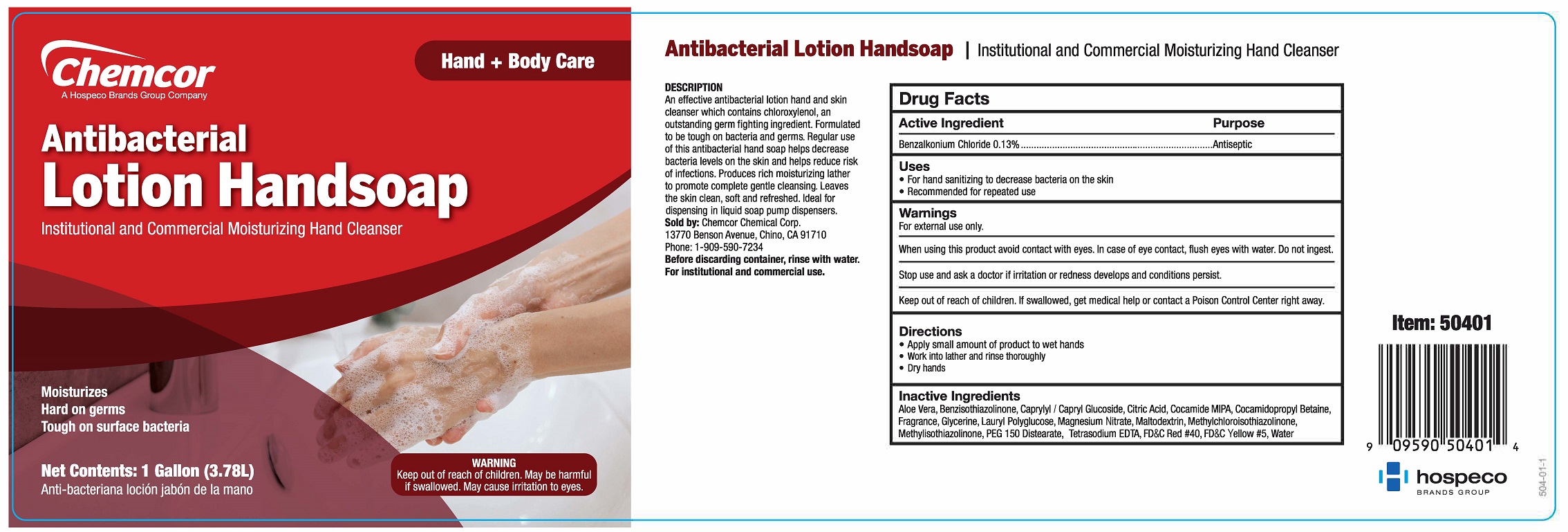
Label: ANTIBACTERIAL HANDSOAP- benzalkonium chloride liquid
- NDC Code(s): 68041-604-01, 68041-604-55
- Packager: Chemcor Chemical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Caprylyl/Capryl Glucoside, Citric Acid, Cocamide MIPA, Cocamidopropyl Betaine, Fragrance, Glycerin, Lauryl Polyglucose, Magnesium Nitrate, Maltodextrin, Methylchloroisothiazolinone, Methylisothiazolinone, PEG 150 Distearate, FD&C Red #40, Tetrasodium EDTA, Water, FD&C Yellow #5
-
SPL UNCLASSIFIED SECTION
moisturizes
hard on germs
tough on surface bacteria
MAY BE HARMFUL IF SWALLOWED.
MAY CAUSE IRRITATION TO EYES.
SEE BACK PANEL FOR ADDITIONAL PRECAUTIONS AND FIRST AID.
institutional & commercial antibacterial hand cleanser
DESCRIPTION
AN EFFECTIVE ANTIBACTERIAL LOTION HAND AND SKIN CLEANSER WHICH CONTAINS CHLOROXYLENOL, AN OUTSTANDING GERM FIGHTING INGREDIENT. FORMULATED TO BE TOUGH ON BACTERIA AND GERMS. REGULAR USE OF THIS ANTIBACTERIAL HAND SOAP HELPS DECREASE BACTERIA LEVELS ON THE SKIN AND HELPS REDUCE RISK OF INFECTIONS. PRODUCES RICH MOISTURIZING LATHER TO PROMOTE COMPLETE GENTLE CLEANSING. LEAVES THE SKIN CLEAN, SOFT AND REFRESHED. IDEAL FOR DISPENSING IN LIQUID SOAP PUMP DISPENSERS.
Before discarding container, rinse with water.
For institutional and commercial use.
- Antibacterial Lotion Handsoap
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL HANDSOAP
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68041-604 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE .13 g in .1 L Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERIN (UNII: PDC6A3C0OX) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) MAGNESIUM NITRATE (UNII: 77CBG3UN78) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) FD&C RED NO. 40 (UNII: WZB9127XOA) EDETATE SODIUM (UNII: MP1J8420LU) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68041-604-01 3.78 L in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2023 2 NDC:68041-604-55 207.9 L in 1 DRUM; Type 0: Not a Combination Product 02/02/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 02/02/2023 Labeler - Chemcor Chemical Corporation (018129978) Establishment Name Address ID/FEI Business Operations Morgan Gallacher Inc. DBA Custom Chemical Formulators Inc. 028311595 manufacture(68041-604) , api manufacture(68041-604) , pack(68041-604)