Label: SULFASALAZINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 10544-288-30 - Packager: Blenheim Pharmacal, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0603-5801
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 25, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
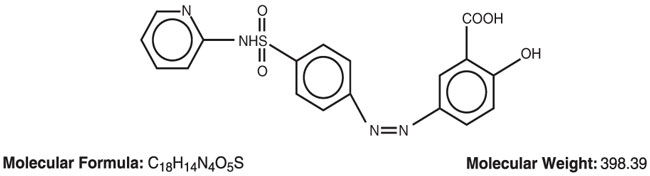
DESCRIPTIONSulfasalazine Tablets USP, 500 mg for oral administration. Therapeutic Classification: Anti-inflammatory agent. Chemical Designation: 5-([ p-(2-Pyridylsulfamoyl)phenyl]azo ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - The mode of action of sulfasalazine (SSZ) or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the ...
-
INDICATIONS AND USAGESulfasalazine tablets, USP are indicated: in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis; and - for the prolongation of the ...
-
CONTRAINDICATIONSSulfasalazine tablets are contraindicated in: Patients with intestinal or urinary obstruction, Patients with porphyria as sulfonamides have been reported to precipitate an acute ...
-
WARNINGSOnly after critical appraisal should sulfasalazine tablets be given to patients with hepatic or renal damage or blood dyscrasias. Deaths associated with the administration of sulfasalazine have ...
-
PRECAUTIONSGeneral: Sulfasalazine tablets should be given with caution to patients with severe allergy or bronchial asthma. Adequate fluid intake must be maintained in order to prevent crystalluria and ...
-
ADVERSE REACTIONSThe most common adverse reactions associated with sulfasalazine are anorexia, headache, nausea, vomiting, gastric distress, and apparently reversible oligospermia. These occur in about one-third ...
-
DRUG ABUSE AND DEPENDENCENone reported.
-
OVERDOSAGEThere is evidence that the incidence and severity of toxicity following overdosage are directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include nausea ...
-
DOSAGE AND ADMINISTRATIONThe dosage of sulfasalazine tablets should be adjusted to each individual's response and tolerance. Initial Therapy: Adults: 3 to 4 g daily in evenly divided doses with dosage intervals not ...
-
HOW SUPPLIEDSulfasalazine Tablets USP, 500 mg are round, gold-colored, scored tablets, debossed "5904" and "V" on one side and plain on the reverse side. They are available in the following package ...
-
REFERENCESMogadam M, et al. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterology 1981;80:72–6. Kaufman DW, editor. Birth defects and ...
-
SPL UNCLASSIFIED SECTIONManufactured for: QUALITEST PHARMACEUTICALS - Huntsville, AL 35811 - 8181485 - Rev 3/14 - R7
-
Principal Display PanelSulfazine® 500mg (Sulfasalazine Tablets, USP 500mg) 30 Tablets - NDC 10544-288-30
-
INGREDIENTS AND APPEARANCEProduct Information