Label: CHLORDIAZEPOXIDE HYDROCHLORIDE AND CLIDINIUM BROMIDE capsule
- NDC Code(s): 59651-524-01
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS and PRECAUTIONS, Drug Interactions).
- The use of benzodiazepines, including chlordiazepoxide hydrochloride, a component of chlordiazepoxide hydrochloride and clidinium bromide capsules, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing chlordiazepoxide hydrochloride and clidinium bromide capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines, including chlordiazepoxide hydrochloride and clidinium bromide capsules, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide hydrochloride and clidinium bromide capsules after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide hydrochloride and clidinium bromide capsules or reduce the dosage (see WARNINGS and DOSAGE AND ADMINISTRATION).
-
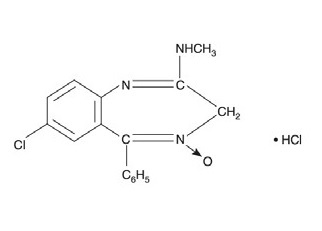
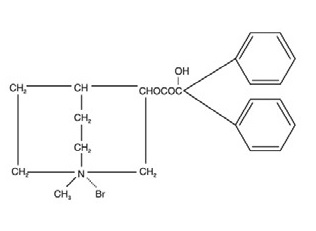
DESCRIPTION:Chlordiazepoxide hydrochloride and clidinium bromide capsules, USP are a fixed-combination of chlordiazepoxide hydrochloride, a benzodiazepine, and clidinium bromide, an anticholinergic. Each ...
-
ANIMAL PHARMACOLOGY AND/OR ANIMAL TOXICOLOGY:Effects on Reproduction - Reproduction studies in rats fed chlordiazepoxide hydrochloride, 10, 20 and 80 mg/kg daily (2.4, 4.8 and 19.4 times, respectively, the maximum recommended clinical dose ...
-
INDICATIONS AND USAGE:Chlordiazepoxide hydrochloride and clidinium bromide capsules are indicated to control emotional and somatic factors in gastrointestinal disorders. Chlordiazepoxide hydrochloride and clidinium ...
-
CONTRAINDICATIONS:Chlordiazepoxide hydrochloride and clidinium bromide capsules are contraindicated in the presence of glaucoma (since the anticholinergic component may produce some degree of mydriasis) and in ...
-
WARNINGS:Risks From Concomitant Use with Opioids - Concomitant use of benzodiazepines, including chlordiazepoxide hydrochloride and clidinium bromide, and opioids may result in profound sedation ...
-
PRECAUTIONS:CNS Adverse Reactions - In geriatric or debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia, oversedation or ...
-
ADVERSE REACTIONS:No side effects or manifestations not seen with either compound alone have been reported with the administration of chlordiazepoxide hydrochloride and clidinium bromide. However, since ...
-
DRUG ABUSE AND DEPENDENCE:Controlled Substance - Chlordiazepoxide hydrochloride and clidinium bromide capsules contain chlordiazepoxide hydrochloride, a Schedule IV controlled substance and clidinium bromide, which is not ...
-
OVERDOSAGEOverdosage of chlordiazepoxide hydrochloride and clidinium bromide, which contains a benzodiazepine (chlordiazepoxide hydrochloride) and an anticholinergic (clidinium bromide) may manifest signs ...
-
DOSAGE AND ADMINISTRATION:Recommended Dosage - Because of the varied individual responses to tranquilizers and anticholinergics, the optimum dosage of chlordiazepoxide hydrochloride and clidinium bromide capsules varies ...
-
HOW SUPPLIED:Chlordiazepoxide Hydrochloride and Clidinium Bromide Capsules USP, 5 mg/2.5 mg are available in green cap and green body hard gelatin capsule shells, imprinted with ‘CH/CB’ on cap, ‘5/2.5’ on ...
-
MEDICATION GUIDEMEDICATION GUIDE - Chlordiazepoxide Hydrochloride and Clidinium Bromide Capsules, USP - (klor-dye-az-e-POX-ide hye-droe-KLOR-ide and kli di' nee um broh myde) for oral use - What is ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg/2.5 mg (100 Capsules Bottle)NDC 59651-524-01 - Chlordiazepoxide - Hydrochloride and Clidinium - Bromide Capsules, USP - 5 mg/2.5 mg - PHARMACIST: Dispense the Medication - Guide provided separately to each patient ...
-
INGREDIENTS AND APPEARANCEProduct Information