Label: ALTACE- ramipril capsule
- NDC Code(s): 61570-110-01, 61570-111-01, 61570-112-01, 61570-120-01
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALTACE safely and effectively. See full prescribing information for ALTACE. ALTACE® (ramipril) capsules, for oral use - Initial ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE1.1 Hypertension - ALTACE is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - The recommended initial dose for patients not receiving a diuretic is 2.5 mg once a day. Adjust dose according to blood pressure response. The usual maintenance dosage range is ...
-
3 DOSAGE FORMS AND STRENGTHSALTACE (ramipril) is supplied as hard gelatin capsules containing 1.25 mg, 2.5 mg, 5 mg, and 10 mg of ramipril.
-
4 CONTRAINDICATIONSALTACE is contraindicated in patients who are hypersensitive to this product or any other ACE inhibitor (e.g., a patient who has experienced angioedema during therapy with any other ACE ...
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylactoid and Possibly Related Reactions - Presumably because drugs that act directly on the renin-angiotensin-aldosterone system (e.g., ACE inhibitors) affect the metabolism of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Diuretics - Patients on diuretics, especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity ...
-
10 OVERDOSAGESingle oral doses of ramipril in rats and mice of 10 g/kg–11 g/kg resulted in significant lethality. In dogs, oral doses as high as 1 g/kg induced only mild gastrointestinal distress. Limited data ...
-
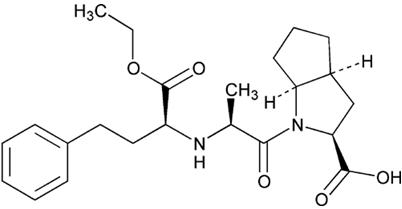
11 DESCRIPTIONRamipril is a 2-aza-bicyclo [3.3.0]-octane-3-carboxylic acid derivative. It is a white, crystalline substance soluble in polar organic solvents and buffered aqueous solutions. Ramipril melts ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ramipril and ramiprilat inhibit ACE in human subjects and animals. Angiotensin converting enzyme is a peptidyl dipeptidase that catalyzes the conversion of angiotensin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of a tumorigenic effect was found when ramipril was given by gavage to rats for up to 24 months at doses of up to 500 ...
-
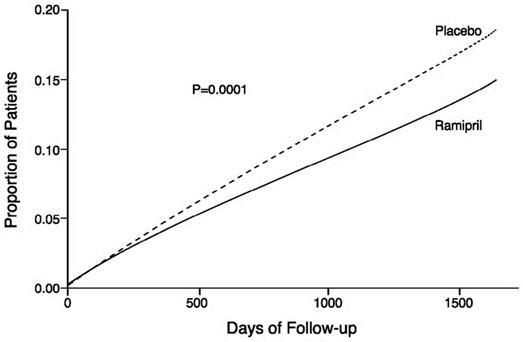
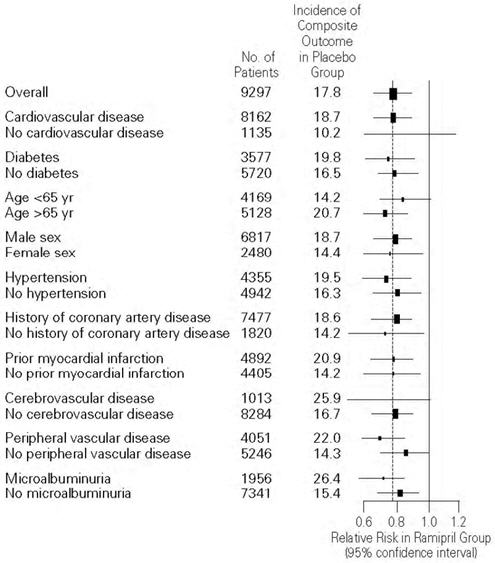
14 CLINICAL STUDIES14.1 Hypertension - ALTACE has been compared with other ACE inhibitors, beta-blockers, and thiazide diuretics as monotherapy for hypertension. It was approximately as effective as other ACE ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGALTACE is available in 1.25 mg, 2.5 mg, 5 mg, and 10 mg hard gelatin capsules. Descriptions of ALTACE capsules are summarized below. Capsule StrengthCapsule ColorPackage ...
-
17 PATIENT COUNSELING INFORMATIONAngioedema - Angioedema, including laryngeal edema, can occur with treatment with ACE inhibitors, especially following the first dose. Advise patients to immediately report any signs or ...
-
SPL UNCLASSIFIED SECTIONLAB-0581-9.0
-
PRINCIPAL DISPLAY PANEL - 1.25 mg Capsule Bottle LabelNDC 61570-110-01 - Pfizer - Altace® (ramipril) capsules, for oral use - 1.25 mg - 100 Capsules - Rx only
-
PRINCIPAL DISPLAY PANEL - 2.5 mg Capsule Bottle LabelNDC 61570-111-01 - Pfizer - Altace® (ramipril) capsules, for oral use - 2.5 mg - 100 Capsules - Rx only
-
PRINCIPAL DISPLAY PANEL - 5 mg Capsule Bottle LabelNDC 61570-112-01 - Pfizer - Altace® (ramipril) capsules, for oral use - 5 mg - 100 Capsules - Rx only
-
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle LabelNDC 61570-120-01 - Pfizer - Altace® (ramipril) capsules, for oral use - 10 mg - 100 Capsules - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information