Label: GOPRELTO- cocaine hydrochloride solution
- NDC Code(s): 70839-359-04
- Packager: LXO US Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GOPRELTO safely and effectively. See full prescribing information for GOPRELTO. GOPRELTO (cocaine hydrochloride) nasal ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence [see Warning and Precautions (5.1). ]
Close -
1 INDICATIONS AND USAGE
GOPRELTO (cocaine hydrochloride) nasal solution is indicated for the induction of local anesthesia of the mucous membranes when performing diagnostic procedures and surgeries on or through the ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions - • GOPRELTO is for intranasal use only. • Do not apply GOPRELTO to damaged nasal mucosa. 2.2 Dosing Recommendation for ...
-
3 DOSAGE FORMS AND STRENGTHS
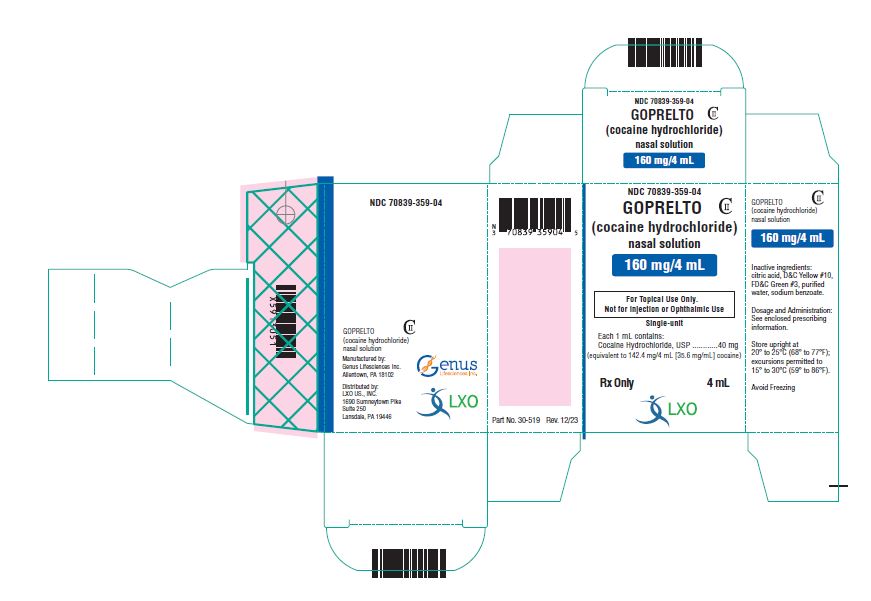
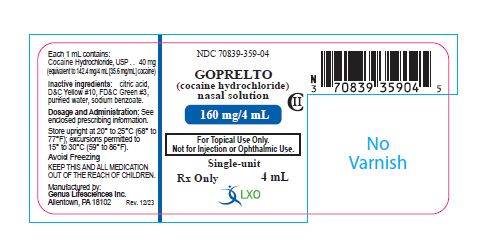
GOPRELTO (cocaine hydrochloride) nasal solution is provided as a 4% solution, 160 mg/4 mL (40 mg/mL), equivalent to 142.4 mg/4 mL (35.6 mg/mL) cocaine, and is a clear, greencolored solution in a ...
-
4 CONTRAINDICATIONS
GOPRELTO is contraindicated in patients with a known history of hypersensitivity to cocaine hydrochloride, other ester-based anesthetics, or any other component of the product.
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence - Central nervous system (CNS) stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence [ see Drug Abuse and ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Disulfiram - Published literature reported that disulfiram treatment increased plasma cocaine exposure, including both AUC and C - max, by several fold after acute intranasal cocaine ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - In animal studies conducted in accordance with good laboratory practices, malformations including vertebral and rib abnormalities were reported when pregnant ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - GOPRELTO contains cocaine, a Schedule II controlled substance. 9.2 Abuse - GOPRELTO contains cocaine, a substance with a high potential for abuse. GOPRELTO can ...
-
10 OVERDOSAGE
No cases of overdose with GOPRELTO were reported in clinical trials. Blood pressure and heart rate increases were greater with cocaine hydrochloride solution 8% than with GOPRELTO. In the case of ...
-
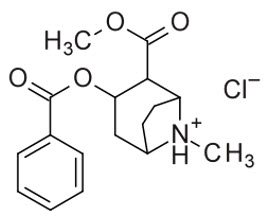
11 DESCRIPTION
GOPRELTO (cocaine hydrochloride) nasal solution for intranasal use contains a 4% solution, 160 mg/4 mL (40 mg/mL), equivalent to 142.4 mg/4 mL (35.6 mg/mL) cocaine, an ester local anesthetic ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Cocaine hydrochloride is a local anesthetic of the ester type. Cocaine hydrochloride prevents conduction in nerve fibers by reversibly blocking sodium channels and ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term animal studies to evaluate the carcinogenic potential of cocaine have not been conducted ...
-
14 CLINICAL STUDIES
A double-blind, multicenter, single-dose, placebo- and dose-controlled, parallel-group study was conducted in 648 subjects undergoing diagnostic procedures and surgeries on or through the mucous ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GOPRELTO (cocaine hydrochloride) nasal solution is a clear, green colored liquid available as one dosage strength: 160 mg/4 mL (40 mg/mL or 4%) cocaine hydrochloride, equivalent to 142.4 mg/4 ...
-
17 PATIENT COUNSELING INFORMATION
Potential for Abuse and Dependence - Advise patients that GOPRELTO is a controlled substance and it can be abused and lead to dependence - [see Warnings and Precautions( 5.1), Drug Abuse ...
-
PRINCIPAL DISPLAY PANELNDC 70839-359-04 - GOPRELTO - CII - (cocaine hydrochloride) nasal solution - 160 mg/4 mL - For Topical Use Only. Not for Injection or Ophthalmic Use - Single-unit - Each 1 mL contains ...
-
INGREDIENTS AND APPEARANCEProduct Information