Label: AQNEURSA- levacetylleucine granule, for suspension
- NDC Code(s): 83853-101-01
- Packager: IntraBio Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AQNEURSA safely and effectively. See full prescribing information for AQNEURSA. AQNEURSA™ (levacetylleucine) for oral suspension ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAQNEURSA™ is indicated for the treatment of neurological manifestations of Niemann-Pick disease type C (NPC) in adults and pediatric patients weighing ≥15 kg.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Recommendation Prior to AQNEURSA Treatment Initiation - For females of reproductive potential, verify that the patient is not pregnant [see Use in Specific Populations (8.1 ...
-
3 DOSAGE FORMS AND STRENGTHSFor oral suspension: 1 gram levacetylleucine as white to off-white strawberry flavored granules in a unit-dose packet.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - Based on findings from animal reproduction studies, AQNEURSA may cause embryo-fetal harm when administered during pregnancy. Administration of levacetylleucine to ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on AQNEURSA - N-acetyl-DL-leucine and N-acetyl-D-leucine - Avoid concomitant use of AQNEURSA with N-acetyl-DL-leucine and N-acetyl-D-leucine. The D-enantiomer ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, AQNEURSA may cause embryo-fetal harm when administered during pregnancy. In animal reproduction studies, an ...
-
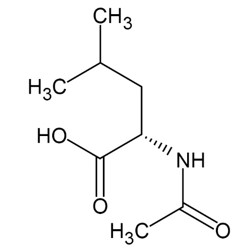
11 DESCRIPTION AQNEURSA (levacetylleucine) for oral suspension contains the drug substance levacetylleucine, a modified amino acid. Levacetylleucine is slightly soluble in aqueous solutions. The chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The distinct molecular target for levacetylleucine in the treatment of NPC is unknown. 12.2 Pharmacodynamics - Clinical pharmacodynamic studies have not been ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal studies to evaluate the carcinogenic potential of levacetylleucine have not been ...
-
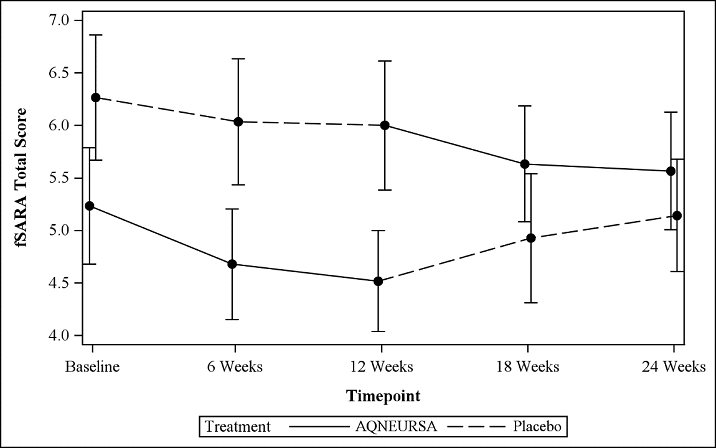
14 CLINICAL STUDIESThe safety and efficacy of AQNEURSA for the treatment of NPC were evaluated in a randomized, double-blind, placebo-controlled, two-period crossover study (NCT05163288) that evaluated the efficacy ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - AQNEURSA (levacetylleucine) for oral suspension is supplied as white to off-white granules in a unit-dose multi-layer aluminum/polyethylene packet. Each packet contains 1.7 gram ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient and/or caregiver to read the FDA-approved patient labeling (Instructions for Use). Embryo-Fetal Toxicity - AQNEURSA may cause embryo-fetal harm. Advise a pregnant female of the ...
-
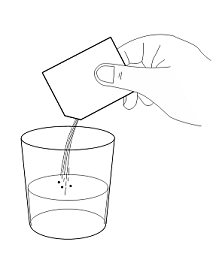
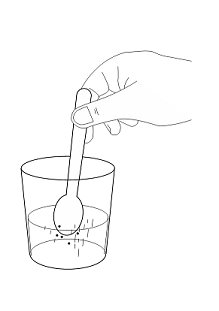
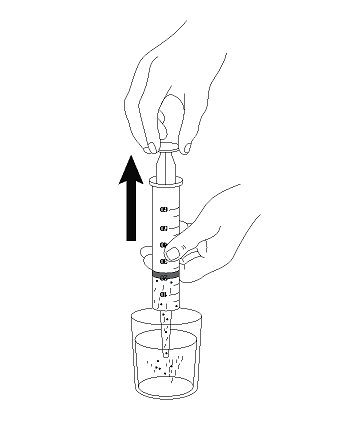
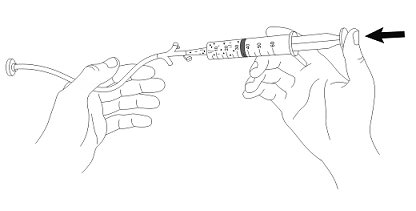
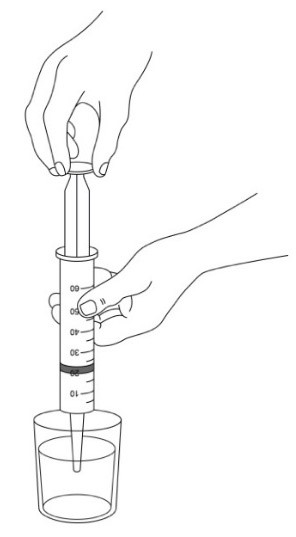
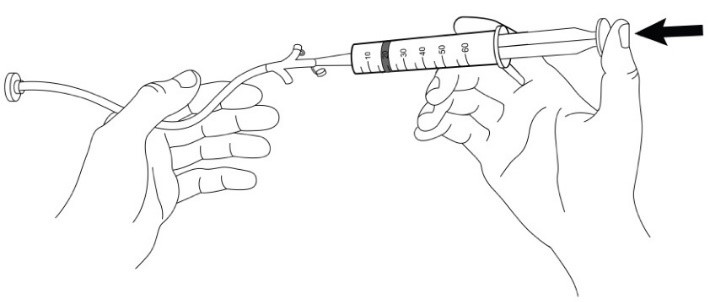
INSTRUCTIONS FOR USE AQNEURSA [ak nur' sah] (levacetylleucine) for oral suspension - This Instructions for Use contains information on how to prepare and take or give AQNEURSA oral suspension. Read this ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information