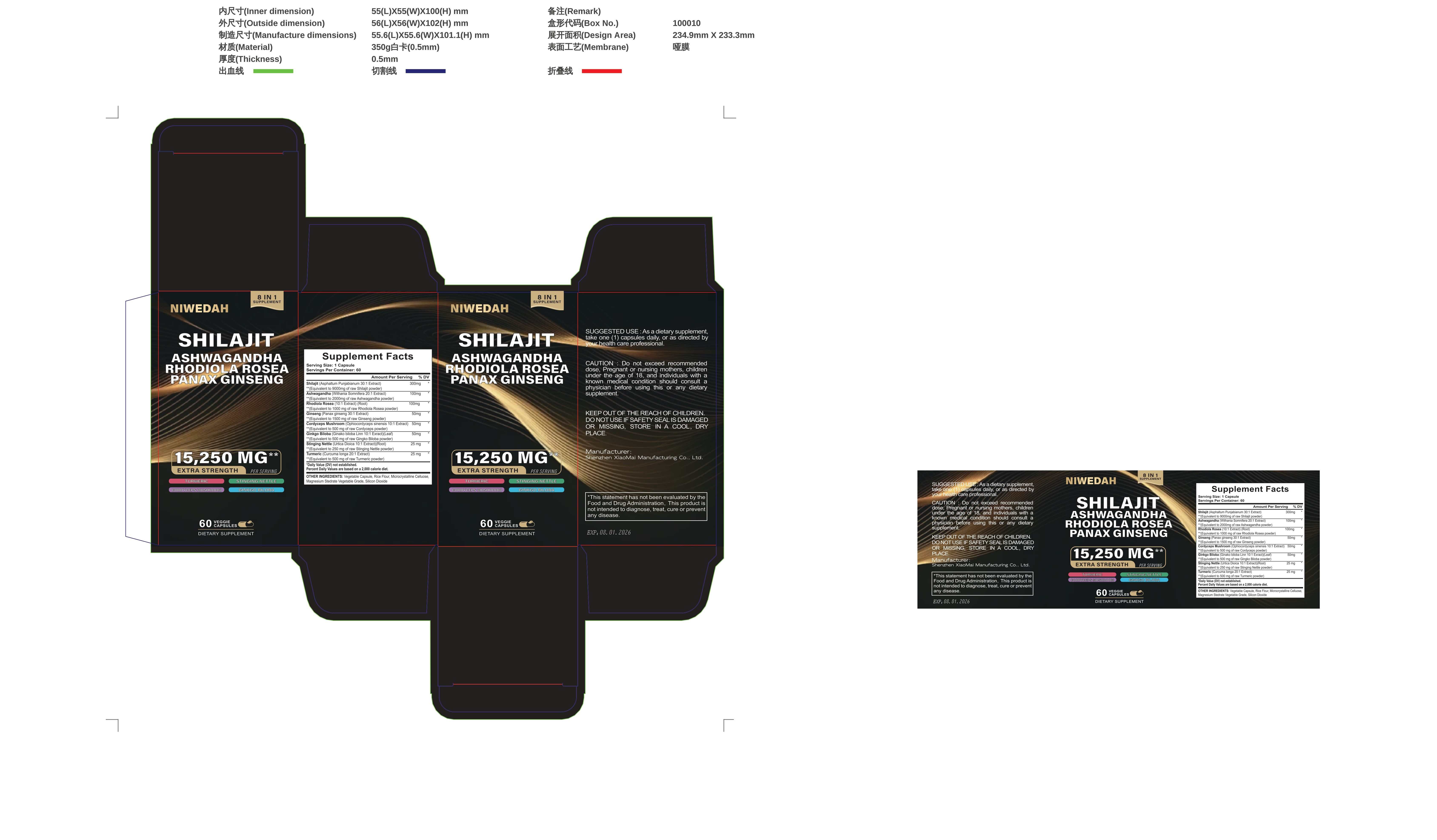
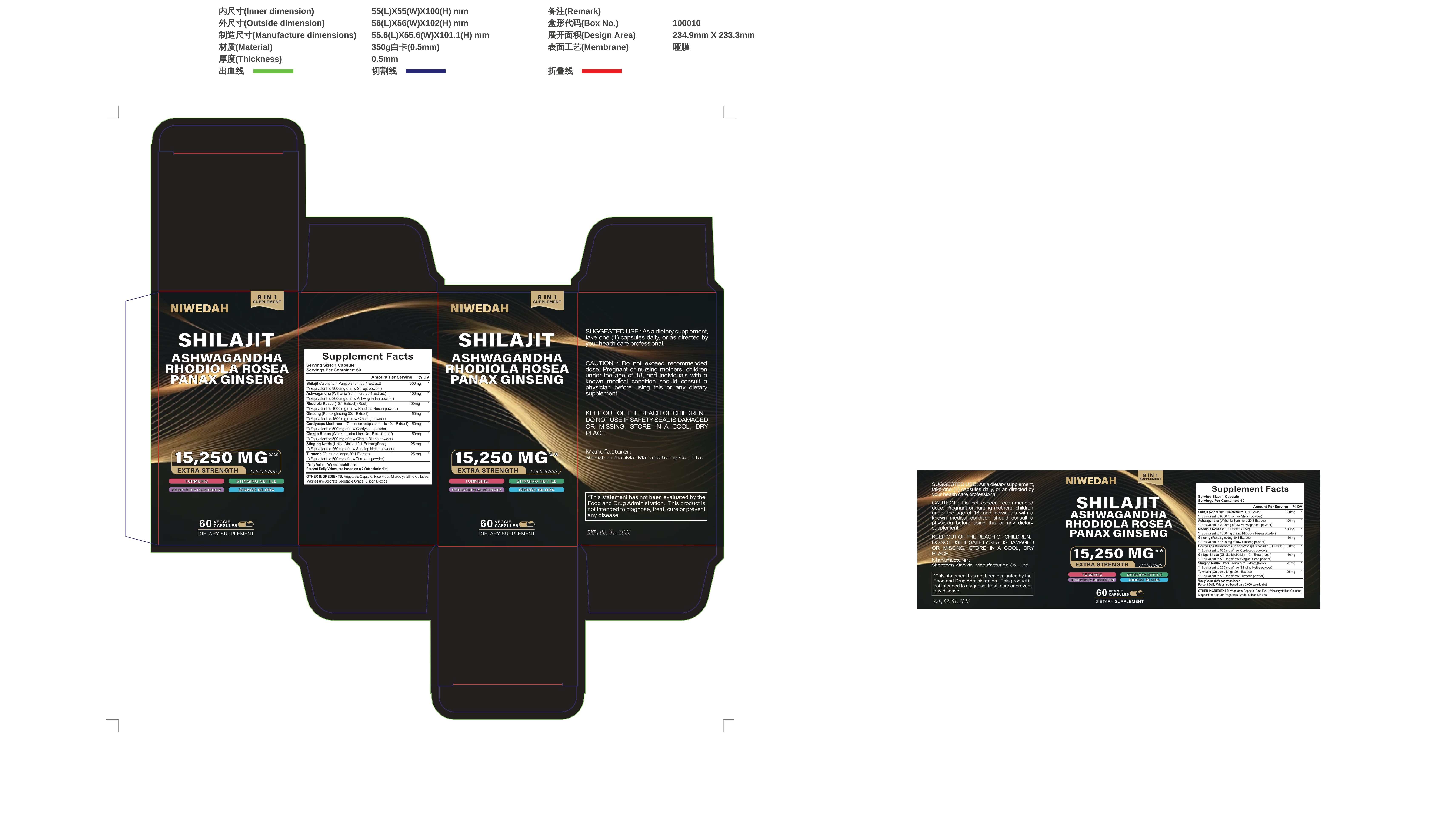
Label: NIWEDAH SHILAJIT ASHWAGANDHA RHODIOLA ROSEA PANAXGINSENG- gieng compound capsulens capsule
- NDC Code(s): 83872-998-01
- Packager: Shenzhen Xiaomai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients
Amount Per Serving% DV
Vitamin C (as Ascorbic Acid) 90 mg 100%
Vitamin D3 (as Cholecalciferol) (2000 IU)25 mog 125%Black Seed (Nigella Sativa 20:1Extract)200mg
Ashwagandha (Withania Somnifera 20:1Extract)100mg
Turmeric (Curcuma Longa 20:1 Extract)(Root)100mg
Bladderwrack (Fucus Vesiculosus 20:1Extract) (Whole Plant)100mg
Burdock (Arctium 20:1 Extract)(Root)100mg
Multimineral Blend350 mg
Multimineral Blend 350 mg
Apple Cider Vinegar (Malus domestica) (Fruit) Powder,
Dandelion (Taraxacum
officinale) (Root) Extract,Yellow Dock (Rumex crispus) (Root) Powder, Eldertberry
Sambucus willamsi hance)(Fruit) Extract,
Manuka Honey (Leptospemum
scoparium)(Root) Powder, Ginger (Zingiber officinale), Chlorophyll.
Black Pepper (Piper nigrum) (Fruit) Extract 5 mg - Purpose
- Uses
- Wamings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Ask Doctor
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIWEDAH SHILAJIT ASHWAGANDHA RHODIOLA ROSEA PANAXGINSENG
gieng compound capsulens capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-998 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASIAN GINSENG (UNII: CUQ3A77YXI) (ASIAN GINSENG - UNII:CUQ3A77YXI) ASIAN GINSENG 5 g in 100 mg Inactive Ingredients Ingredient Name Strength WITHANIA COAGULANS ROOT (UNII: 73TQY6I31J) ELAPHOCORDYCEPS OPHIOGLOSSOIDES WHOLE (UNII: 932F61H68V) GINKGO BILOBA WHOLE (UNII: 660486U6OI) CHLOROPHYLL A (UNII: YF5Q9EJC8Y) AMINO ACIDS, RICE (UNII: 5ET1T25H82) TURMERIC (UNII: 856YO1Z64F) RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) RHODIOLA CRENULATA ROOT (UNII: G7TMD86VH5) Product Characteristics Color blue Score no score Shape CAPSULE Size 112mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-998-01 15250 mg in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/14/2024 Labeler - Shenzhen Xiaomai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen Xiaomai Manufacturing Co., Ltd. 712999147 manufacture(83872-998)