Label: OSMITROL- mannitol injection, solution
-
NDC Code(s):
0338-0351-04,
0338-0353-03,
0338-0355-03,
0338-0357-02, view more0338-0357-03
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OSMITROL safely and effectively. See full prescribing information for OSMITROL.
OSMITROL (mannitol injection), for intravenous use
Initial U.S. Approval: 1964RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Administration Instructions (2.1):
- •
- For intravenous infusion preferably into a large central vein.
- •
- Prior to administration, evaluate renal, cardiac and pulmonary status, and correct fluid and electrolyte imbalances.
Recommended Dosage (2.2):
- •
- The dosage, concentration and rate of administration depend on the age, weight and condition of the patient, including fluid requirement, urinary output and concomitant therapy.
- •
- Reduction of Intracranial Pressure: 0.25 gram/kg administered every 6 to 8 hours as an intravenous infusion over 30 minutes.
- •
- Reduction of Intraocular Pressure: 1.5 to 2 grams/kg of a 15% or 20% w/v solution as a single dose administered intravenously over at least 30 minutes.
DOSAGE FORMS AND STRENGTHS
Injection (3):
- •
- 5% (0.05 grams/mL): 5 grams of mannitol, USP per 100 mL in a single-dose 1000 mL flexible container
- •
- 10% (0.1 grams/mL): 10 grams of mannitol, USP per 100 mL in a single-dose 500 mL flexible container
- •
- 15% (0.15 grams/mL): 15 grams of mannitol, USP per 100 mL in a single-dose 500 mL flexible container
- •
- 20% (0.2 grams/mL): 20 grams of mannitol, USP per 100 mL in single-dose 250 mL and 500 mL flexible containers
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity Reactions, including anaphylaxis: Stop infusion immediately if hypersensitivity reactions develop. (5.1)
- •
- Renal Complications Including Renal Failure: Risk factors include pre-existing renal failure, concomitant use of nephrotoxic drugs or other diuretics. Avoid use of nephrotoxic drugs. Discontinue OSMITROL if renal function worsens. (5.2, 8.6)
- •
- Central Nervous System (CNS) Toxicity: Confusion, lethargy and coma may occur during or after infusion. Concomitant neurotoxic drugs may potentiate toxicity. Avoid use of neurotoxic drugs. Discontinue OSMITROL if CNS toxicity develops. (5.3)
- •
- Fluid and Electrolyte Imbalances, Hyperosmolarity: Hypervolemia may exacerbate congestive heart failure, hyponatremia can lead to encephalopathy; hypo/hyperkalemia can result in cardiac adverse reactions in sensitive patients. Discontinue OSMITROL if fluid and/or electrolyte imbalances occur. (5.4)
- •
- Monitoring/Laboratory Tests: Monitor fluid and electrolytes, serum osmolarity and renal, cardiac and pulmonary function. Discontinue if toxicity develops. (5.5)
- •
- Infusion Site Reactions: May include irritation and inflammation, as well as severe reactions (compartment syndrome) when associated with extravasation. (5.6)
- •
- Interference with Laboratory Tests: High concentrations of mannitol may cause false low results of inorganic phosphorus blood concentrations. Mannitol may produce false positive results for blood ethylene glycol. (5.7, 7.6)
ADVERSE REACTIONS
- The most common adverse reactions are hypersensitivity reactions, renal failure, CNS toxicity, hypo/hypervolemia, hypo/hypernatremia, hypo/hyperkalemia, and infusion site reactions. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Nephrotoxic Drugs and Diuretics: May increase the risk of renal failure; avoid concomitant use. (7.1, 7.2)
- •
- Neurotoxic Drugs: May potentiate CNS toxicity of mannitol; avoid concomitant use. (7.3)
- •
- Drugs Affected by Electrolyte Imbalances: May result in cardiac adverse reactions; monitor serum electrolytes and discontinue OSMITROL if cardiac status worsens. (7.4)
- •
- Renally Eliminated Agents: Concomitant use may decrease the effectiveness of agents that undergo significant renal elimination. However, concomitant use of mannitol and lithium may increase risk of lithium toxicity. If concomitant use is necessary, frequently monitor lithium concentrations and for signs of toxicity. (7.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Renal Complications Including Renal Failure
5.3 Central Nervous System (CNS) Toxicity
5.4 Fluid and Electrolyte Imbalances, Hyperosmolarity
5.5 Monitoring/Laboratory Tests
5.6 Infusion Site Reactions
5.7 Interference with Laboratory Tests
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Nephrotoxic Drugs
7.2 Diuretics
7.3 Neurotoxic Drugs
7.4 Drugs Affected by Electrolyte Imbalances
7.5 Renally Eliminated Drugs
7.6 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- •
- OSMITROL is for intravenous infusion preferably into a large central vein [see Warnings and Precautions (5.5), Description (11)].
- •
- Prior to administration of OSMITROL, evaluate renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances [see Dosage and Administration (2.2)].
- •
- Do not administer OSMITROL simultaneously with blood products through the same administration set because of the possibility of pseudoagglutination or hemolysis.
Preparation
- 1.
- Tear overwrap down side at slit and remove solution container.
- 2.
- Visually inspect the container. Do not administer unless solution is clear and seal is intact.
- o
- If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired.
- o
- Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
- 3.
- Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
- 4.
- Admixing OSMITROL with other medications is not recommended.
- 5.
- Inspect OSMITROL visually for particulate matter and discoloration prior to administration. If particulates or discoloration are present, discard the bag.
- 6.
- OSMITROL solutions may crystallize when exposed to low temperature. At higher concentrations, the solutions have a greater tendency to crystallize. Inspect OSMITROL for crystals prior to administration. If crystals are visible, re-dissolve by warming the solution up to 70°C, with agitation. Solutions should not be heated in water or in a microwave oven due to potential for product contamination or damage. Allow the solution to cool to room or body temperature before re-inspection for crystals and use.
Administration
- 1.
- Suspend container from eyelet support.
- 2.
- Remove protector from outlet port at bottom of container.
- 3.
- Attach administration set. Refer to complete directions accompanying set.
- 4.
- Use administration sets with a final in-line filter because of the potential for OSMITROL crystals to form.
- 5.
- To prevent air embolism, use a non-vented infusion set or close the vent on a vented set, avoid multiple connections, do not connect flexible containers in series, fully evacuate residual gas in the container prior to administration, do not pressurize the flexible container to increase flow rates, and if administration is controlled by a pumping device, turn off pump before the container runs dry.
- 6.
- For single use only; discard unused portion.
2.2 Recommended Dosage
Prior to administration of OSMITROL, evaluate renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances.
The total dosage, concentration, and rate of administration depend on the age, weight, and condition of the patient being treated, including fluid requirement, electrolyte balance, serum osmolality, urinary output, and concomitant therapy.
The following outline of administration and dosage is only a general guide to therapy.
Reduction of Intracranial Pressure
Usually a maximum reduction in intracranial pressure can be achieved with a dose of 0.25 gram/kg given intravenously as an intravenous infusion over 30 minutes which may be repeated every six to eight hours.During and following OSMITROL infusion, monitor fluid and electrolytes, serum osmolarity, and renal, cardiac and pulmonary function. Discontinue OSMITROL if renal, cardiac, or pulmonary status worsens or CNS toxicity develops [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
Reduction of Intraocular Pressure
The recommended dosage is 1.5 to 2 grams/kg of a 20% w/v solution (7.5 to 10 mL/kg) or as a 15% w/v solution (10 to 13 mL/kg) as a single dose administered intravenously over at least 30 minutes. When used preoperatively, administer OSMITROL sixty to ninety minutes before surgery to achieve maximal reduction of intraocular pressure before operation.
-
3 DOSAGE FORMS AND STRENGTHS
OSMITROL Injection:
- •
- 5% (0.05 grams/mL): 5 grams of mannitol, USP per 100 mL in a single-dose 1000 mL flexible container
- •
- 10% (0.1 grams/mL): 10 grams of mannitol, USP per 100 mL in a single-dose 500 mL flexible container
- •
- 15% (0.15 grams/mL): 15 grams of mannitol, USP per 100 mL in a single-dose 500 mL flexible container
- •
- 20% (0.2 grams/mL): 20 grams of mannitol, USP per 100 mL in single-dose 250 mL and 500 mL flexible containers
-
4 CONTRAINDICATIONS
OSMITROL is contraindicated in patients with:
- •
- Known hypersensitivity to mannitol [see Warnings and Precautions (5.1)]
- •
- Anuria [see Warnings and Precautions (5.2)]
- •
- Severe hypovolemia [see Warnings and Precautions (5.4)]
- •
- Pre-existing severe pulmonary vascular congestion or pulmonary edema [see Warnings and Precautions (5.5)]
- •
- Active intracranial bleeding except during craniotomy
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, hypotension and dyspnea resulting in cardiac arrest and death have been reported with OSMITROL [see Adverse Reactions (6)].
Stop the infusion immediately if signs or symptoms of a suspected hypersensitivity reaction develop. Initiate appropriate therapeutic countermeasures as clinically indicated.
5.2 Renal Complications Including Renal Failure
Renal complications, including irreversible renal failure have been reported in patients receiving mannitol. Reversible, oliguric acute kidney injury (AKI) has occurred in patients with normal pretreatment renal function who received large intravenous doses of mannitol. Although the osmotic nephrosis associated with mannitol administration is in principle reversible, osmotic nephrosis in general is known to potentially proceed chronic or even end-stage renal failure. Monitor renal function closely, including signs of urine output reduction, during OSMITROL infusion. Patients with pre-existing renal disease, patients with conditions that put them at high risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure following administration of OSMITROL. Avoid concomitant administration of nephrotoxic drugs (e.g., aminoglycosides) or, other diuretics with OSMITROL, if possible [see Drug Interactions (7.1, 7.2)].
Patients with oliguric AKI who subsequently develop anuria while receiving mannitol are at risk of congestive heart failure, pulmonary edema, hypertensive crisis, coma and death.
During and following OSMITROL infusion for the reduction in intracranial pressure, monitor the patient clinically and laboratory tests for changes in fluid and electrolyte status. Discontinue OSMITROL if renal function worsens. [see Warnings and Precautions (5.5)].
5.3 Central Nervous System (CNS) Toxicity
CNS toxicity manifested by, e.g., confusion, lethargy, coma has been reported in patients treated with mannitol, some resulting in death, in particular in the presence of impaired renal function CNS toxicity may result from high serum mannitol concentrations, serum hyperosmolarity resulting in intracellular dehydration within CNS, hyponatremia or other disturbances of electrolyte and acid/base balance secondary to mannitol administration [see Warnings and Precautions (5.4)].
At high concentrations, mannitol may cross the blood brain barrier and interfere with the ability of the brain to maintain the pH of the cerebrospinal fluid especially in the presence of acidosis.
In patients with preexisting compromise of the blood brain barrier, the risk of increasing cerebral edema (general and focal) associated with repeated or continued use of OSMITROL must be individually weighed against the expected benefits.
A rebound increase of intracranial pressure may occur several hours after the infusion. Patients with a compromised blood brain barrier are at increased risk.
Concomitant administration of neurotoxic drugs (e.g., aminoglycosides) with OSMITROL may potentiate neurotoxicity. Avoid concomitant use of neurotoxic drugs, if possible [see Drug Interactions (7.3)].
During and following OSMITROL infusion for the reduction in intracranial pressure, monitor the patient clinically and laboratory tests for changes in fluid and electrolyte status. Discontinue OSMITROL if CNS toxicity develops [see Warnings and Precautions (5.5)].
5.4 Fluid and Electrolyte Imbalances, Hyperosmolarity
Depending on dosage and duration, administration of OSMITROL may result in hypervolemia leading to or exacerbating existing congestive heart failure. Accumulation of mannitol due to insufficient renal excretion increases the risk of hypervolemia. Mannitol-induced osmotic diuresis may cause or worsen dehydration/hypovolemia and hemoconcentration. Administration of OSMITROL may also cause hyperosmolarity [see Description (11)].
Depending on dosage and duration of administration, electrolyte and acid/base imbalances may also result from transcellular shifts in water and electrolytes, osmotic diuresis and/or other mechanisms. Such imbalances may be severe and potentially fatal.
Imbalances that may result from OSMITROL administration include:
- •
- Hypernatremia, dehydration and hemoconcentration
- •
- Hyponatremia, which can lead to headache, nausea, seizures, lethargy, coma, cerebral edema, and death. Acute symptomatic hyponatremic encephalopathy is considered a medical emergency.
- •
- Hypo/hyperkalemia. The development of electrolyte imbalances (e.g., hyperkalemia, hypokalemia) associated with mannitol administration may result in cardiac adverse reactions in patients receiving drugs that are sensitive to such imbalances (e.g., digoxin, agents that may cause QT prolongation, neuromuscular blocking agents) [see Drug Interactions (7.4)].
- •
- Other electrolyte disturbances
- •
- Metabolic acidosis/alkalosis
Pediatric patients less than two years of age, particularly preterm and term neonates, may be at higher risk for fluid and electrolyte abnormalities following OSMITROL administration due to decreased glomerular filtration rate and limited ability to concentrate urine [see Use in Specific Populations (8.4)].
During and following OSMITROL infusion for the reduction in intracranial pressure, monitor fluid and electrolyte status and discontinue OSMITROL if imbalances occur [see Warnings and Precautions (5.5)].
5.5 Monitoring/Laboratory Tests
During and following OSMITROL infusion for the reduction in intracranial pressure, monitor:
- •
- serum osmolarity, serum electrolytes (including sodium, potassium, calcium and phosphate) and acid base balance,
- •
- the osmol gap
- •
- signs of hypo- or hypervolemia, including urine output
- •
- renal, cardiac and pulmonary function
- •
- intracranial pressure
Discontinue OSMITROL if renal, cardiac, or pulmonary status worsens or CNS toxicity develops [see Contraindications (4)].
5.6 Infusion Site Reactions
The infusion of hypertonic solutions through a peripheral vein, including OSMITROL at a concentration of 10% w/v or greater, may result in peripheral venous irritation, including phlebitis. Other severe infusion site reactions, such as compartment syndrome and swelling associated with extravasation, can occur with administration of OSMITROL [see Adverse Reactions (6)]. OSMITROL is preferably for administration into a large central vein [see Dosage and Administration (2.1)].
5.7 Interference with Laboratory Tests
High concentrations of mannitol can cause false low results for inorganic phosphorus blood concentrations [see Drug Interactions (7.6)].
Mannitol may produce false positive results in tests for blood ethylene glycol concentrations [see Drug Interactions (7.6)].
-
6 ADVERSE REACTIONS
The following adverse reactions from voluntary reports or clinical studies have been reported with OSMITROL. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Hypersensitivity reactions: cardiac arrest, anaphylaxis, hypotension, dyspnea, hypertension, pyrexia, chills, sweating, cough, musculoskeletal stiffness, myalgia, urticarial/rash, pruritus, generalized pain, discomfort, nausea, vomiting, and headache. [see Warnings and Precautions (5.1)]
- •
- Renal Failure: acute kidney injury, osmotic nephrosis, azotemia, anuria, hematuria, oliguria, polyuria [see Warnings and Precautions (5.2)]
- •
- CNS Toxicity: coma, seizures, confusion, lethargy; rebound increase in intracranial pressure; dizziness [see Warnings and Precautions (5.3)]
- •
- Fluid and Electrolyte Imbalances: hypovolemia, hypervolemia, peripheral edema, dehydration, hyponatremia, hypernatremia, hyperkalemia, hypokalemia; metabolic acidosis [see Warnings and Precautions (5.4)]
- •
- Infusion Site Reactions: phlebitis, inflammation, pain, rash, erythema, pruritus; compartment syndrome and swelling associated with extravasation [see Warnings and Precautions (5.6)]
- •
- Cardiac and Respiratory Disorders: congestive cardiac failure, pulmonary edema, palpitations
- •
- Gastrointestinal Disorders: thirst, dry mouth
- •
- General Disorders: asthenia, malaise
-
7 DRUG INTERACTIONS
7.1 Nephrotoxic Drugs
Concomitant administration of nephrotoxic drugs (e.g., cyclosporine, aminoglycosides) increases the risk of renal failure following administration of mannitol. Avoid use of nephrotoxic drugs with OSMITROL, if possible [see Warnings and Precautions (5.2)].
7.2 Diuretics
Concomitant administration of other diuretics may potentiate the renal toxicity of mannitol. Avoid use of other diuretics with OSMITROL, if possible [see Warnings and Precautions (5.2)].
7.3 Neurotoxic Drugs
Concomitant administration of systemic neurotoxic drugs (e.g., aminoglycosides) with OSMITROL may potentiate the CNS toxicity of mannitol. Avoid use of systemic neurotoxic drugs with OSMITROL, if possible [see Warnings and Precautions (5.3)].
7.4 Drugs Affected by Electrolyte Imbalances
The development of electrolyte imbalances (e.g., hyperkalemia, hypokalemia) associated with mannitol administration may result in cardiac adverse reactions in patients receiving drugs that are sensitive to such imbalances (e.g., digoxin, drugs that prolong the QT interval, neuromuscular blocking agents) [see Warnings and Precautions (5.4)]. During and following OSMITROL infusion, monitor serum electrolytes and discontinue OSMITROL if cardiac status worsens [see Warnings and Precautions (5.5)].
7.5 Renally Eliminated Drugs
Mannitol therapy may increase the elimination, and decrease the effectiveness of treatment with, drugs that undergo significant renal elimination. Concomitant administration of mannitol with lithium may initially increase the elimination of lithium but may also increase the risk of lithium toxicity if patients develop hypovolemia or renal impairment. In patients receiving lithium, consider holding lithium doses during treatment with OSMITROL. In patients requiring concomitant administration of lithium and OSMITROL, frequently monitor serum lithium concentrations and for signs of lithium toxicity.
7.6 Interference with Laboratory Tests
High concentrations of mannitol can cause false low results for inorganic phosphorus blood concentrations when an assay based on the conversion of phosphate (orthophosphate) to the phosphomolybdate complex is used.
Mannitol may produce false positive results in tests for blood ethylene glycol concentrations in which mannitol is initially oxidized to an aldehyde.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available case report data with mannitol over decades of use have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Mannitol crosses the placenta and may cause fluid shifts that could potentially result in adverse effects in the fetus (see Data). No adverse developmental effects from mannitol were reported in published animal studies; however, fluid shifts occurred in fetal ewes in response to maternal infusion of mannitol.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mannitol in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OSMITROL and any potential adverse effects on the breastfed infant from OSMITROL or from the underlying maternal condition.
8.4 Pediatric Use
OSMITROL is approved for use in the pediatric population for the reduction of intracranial and intraocular pressure. Studies have not defined the optimal dose of OSMITROL in the pediatric population. The safety profile for mannitol use in pediatric patients is similar to adults at dosages described in labeling. However, pediatric patients less than two years of age, particularly preterm and term neonates, may be at higher risk for fluid and electrolyte abnormalities following OSMITROL administration due to decreased glomerular filtration rate and limited ability to concentrate urine [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
Mannitol is known to be substantially excreted by the kidney and the risk of adverse reactions to this drug may be greater in elderly patients with impaired renal function. Evaluate the renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of OSMITROL [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
8.6 Renal Impairment
Patients with pre-existing renal disease, patients with conditions that put them at high risk for renal failure, or those receiving potentially nephrotoxic drugs or other diuretics, are at increased risk of renal failure with administration of mannitol. Evaluate the renal, cardiac and pulmonary status of the patient and correct fluid and electrolyte imbalances prior to administration of OSMITROL [see Warnings and Precautions (5.2, 5.3, 5.4, 5.5)].
-
10 OVERDOSAGE
Signs and symptoms of overdose with OSMITROL include renal failure and AKI, hypo/hypervolemia, hyperosmolarity and electrolyte imbalances, CNS toxicity (e.g., coma, seizures), some of which can be fatal [see Warnings and Precautions (5.2, 5.3, 5.4)].
Management of overdosage with OSMITROL is symptomatic and supportive. Discontinue the infusion and institute appropriate corrective measures with particular attention to renal, cardiac and pulmonary systems. Correct fluid and electrolyte imbalances.
OSMITROL is dialyzable (hemodialysis and peritoneal dialysis), hemodialysis may increase OSMITROL elimination.
-
11 DESCRIPTION
OSMITROL is a sterile, nonpyrogenic solution of Mannitol, USP in a single-dose flexible container for intravenous administration as an osmotic diuretic. It contains no antimicrobial agents. Mannitol is a six carbon sugar alcohol prepared commercially by the reduction of dextrose. The pH is adjusted with sodium hydroxide or hydrochloric acid. Composition, osmolarity, and pH are shown in .
- *
- Normal physiologic osmolarity range is approximately 280 to 310 mOsmol/L.
Table 1
Size
Composition
*Osmolarity
pH
(mL)
Mannitol, USP
(g/L)(mOsmol/L)
(calc)5%
OSMITROL1000
50
274
5.0
(4.5 TO 7.0)10%
OSMITROL500
100
549
5.0
(4.5 TO 7.0)15%
OSMITROL500
150
823
5.0
(4.5 TO 7.0)20%
OSMITROL250
200
1098
5.0
(4.5 TO 7.0)500
The plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mannitol, when administered intravenously, exerts its osmotic diuretic effect as a solute of relatively small molecular size being largely confined to the extracellular space. Mannitol hinders tubular reabsorption of water and enhances excretion of sodium and chloride by elevating the osmolarity of the glomerular filtrate.
This increase in extracellular osmolarity affected by the intravenous administration of mannitol will induce the movement of intracellular water to the extracellular and vascular spaces. This action underlies the role of mannitol in reducing intracranial pressure, intracranial edema, and intraocular pressure.
12.3 Pharmacokinetics
Distribution
Mannitol distributes largely in the extracellular space in 20 to 40 minutes after intravenous administration. The volume of distribution of mannitol is approximately 17 L in adults.
Elimination
In subjects with normal renal function, the total clearance is 87 to 109 mL/min. The elimination half-life of mannitol is 0.5 to 2.5 hours.
Metabolism
Only relatively small amount of the dose administered is metabolized after intravenous administration of mannitol to healthy subjects.
Excretion
Mannitol is eliminated primarily via the kidneys in unchanged form. Mannitol is filtered by the glomeruli, exhibits less than 10% of tubular reabsorption, and is not secreted by tubular cells. Following intravenous administration, approximately 80% of an administered dose of mannitol is estimated to be excreted in the urine in three hours with lesser amounts thereafter.
Specific Populations
Patients with Renal Impairment
In patients with renal impairment, the elimination half-life of mannitol is prolonged. In a published study, in patients with renal impairment including acute renal failure and end stage renal failure, the elimination half-life of mannitol was estimated at about 36 hours, based on serum osmolarity. In patients with renal impairment on dialysis, the elimination half-life of mannitol was reduced to 6 and 21 hours during hemodialysis and peritoneal dialysis, respectively. [see Use in Specific Populations (8.6), Overdosage (10)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OSMITROL injection is supplied in single-dose, flexible VIAFLEX plastic containers and is available as follows:
Code
Size (mL)
NDC
Product Name
2D5604
1000
0338-0351-04
5% (0.05 g/mL mannitol, USP)
2D5613
500
0338-0353-03
10% (0.1 g/mL mannitol, USP)
2D5623
500
0338-0355-03
15% (0.15 g/mL mannitol, USP)
2D5632
250
0338-0357-02
20% (0.2 g/mL mannitol, USP)
2D5633
500
0338-0357-03
20% (0.2 g/mL mannitol, USP)
Do not remove container from overwrap until intended for use.
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Store at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
-
17 PATIENT COUNSELING INFORMATION
Inform patients or caregivers of the following risks of OSMITROL:
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- •
- Renal Failure [see Warnings and Precautions (5.2)]
- •
- CNS Toxicity [see Warnings and Precautions (5.3)]
- •
- Fluid and Electrolyte Imbalances, Hyperosmolarity [see Warnings and Precautions (5.4)]
- •
- Infusion Site Reactions [see Warnings and Precautions (5.6)]
- SPL UNCLASSIFIED SECTION
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
2D5604
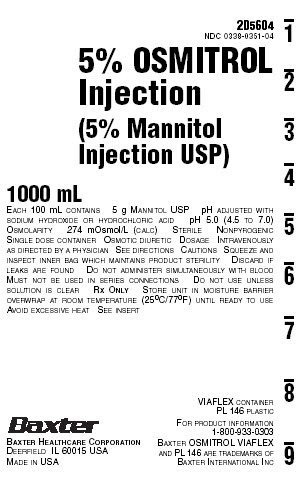
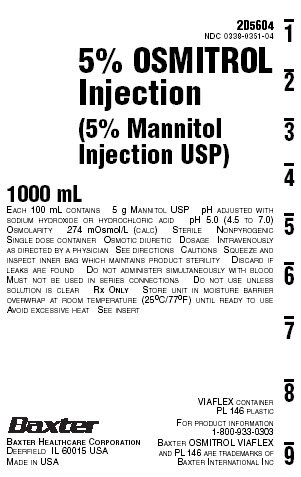
NDC 0338-0351-045% OSMITROL
Injection
(5% Mannitol
Injection USP)1000 mL
EACH 100 mL CONTAINS 5 g MANNITOL USP pH ADJUSTED WITH
SODIUM HYDROXIDE OR HYDROCHLORIC ACID pH 5.0 (4.5 TO 7.0)
OSMOLARITY 274 mOsmol/L (CALC) STERILE NONPYROGENIC
SINGLE DOSE CONTAINER OSMOTIC DIURETIC DOSAGE INTRAVENOUSLY
AS DIRECTED BY A PHYSICIAN SEE DIRECTIONS CAUTIONS SQUEEZE AND
INSPECT INNER BAG WHICH MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND DO NOT ADMINISTER SIMULTANEOUSLY WITH BLOOD
MUST NOT BE USED IN SERIES CONNECTIONS DO NOT USE UNLESS
SOLUTION IS CLEAR RX ONLY STORE UNIT IN MOISTURE BARRIER
OVERWRAP AT ROOM TEMPERATURE (25°C/77ºF) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTVIAFLEX CONTAINER
PL 146 PLASTICFOR PRODUCT INFORMATION
1-800-933-0303BAXTER OSMITROL VIAFLEX
AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCBAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA2D5613
NDC 0338-0353-0310% OSMITROL
Injection
(10% Mannitol
Injection USP)500 mL
EACH 100 mL CONTAINS 10 g MANNITOL USP pH
ADJUSTED WITH SODIUM HYDROXIDE OR HYDROCHLORIC ACID
pH 5.0 (4.5 TO 7.0) HYPERTONIC OSMOLARITY 549
mOsmol/L (CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER OSMOTIC DIURETIC DOSAGE INTRAVENOUSLY
AS DIRECTED BY A PHYSICIAN SEE DIRECTIONS CAUTIONS
SQUEEZE AND INSPECT INNER BAG WHICH MAINTAINS PRODUCT
STERILITY DISCARD IF LEAKS ARE FOUND DO NOT
ADMINISTER SIMULTANEOUSLY WITH BLOOD MUST NOT BE
USED IN SERIES CONNECTIONS DO NOT USE UNLESS
SOLUTION IS CLEAR RX ONLY STORE UNIT IN MOISTURE
BARRIER OVERWRAP AT ROOM TEMPERATURE (25°C/77ºF)
UNTIL READY TO USE AVOID EXCESSIVE HEAT SEE INSERTVIAFLEX CONTAINER
PL 146 PLASTICFOR PRODUCT INFORMATION
1-800-933-0303BAXTER OSMITROL VIAFLEX
AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCBAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA2D5623
NDC 0338-0355-0315% OSMITROL
Injection
(15% Mannitol
Injection USP)500 mL
EACH 100 mL CONTAINS 15 g MANNITOL USP pH ADJUSTED WITH
SODIUM HYDROXIDE OR HYDROCHLORIC ACID pH 5.0 (4.5 TO 7.0)
OSMOLARITY 823 mOsmol/L (CALC) HYPERTONIC MAY CAUSE
VEIN DAMAGE STERILE NONPYROGENIC SINGLE DOSE CONTAINER
OSMOTIC DIURETIC DOSAGE INTRAVENOUSLY AS DIRECTED BY A
PHYSICIAN USING A FILTER TYPE SET SEE DIRECTIONS CAUTIONS
SQUEEZE AND INSPECT INNER BAG WHICH MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND DO NOT ADMINISTER SIMULTANEOUSLY
WITH BLOOD MUST NOT BE USED IN SERIES CONNECTIONS DO NOT
USE UNLESS SOLUTION IS CLEAR IF CRYSTALS ARE VISIBLE REDISSOLVE
BY WARMING UNIT TO 70°C/158°F WITH AGITATION COOL TO ROOM
TEMPERATURE AND REINSPECT FOR CRYSTALS BEFORE INFUSION
RX ONLY STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (25°C/77ºF) UNTIL READY TO USE AVOID EXCESSIVE
HEAT SEE INSERTVIAFLEX CONTAINER
PL 146 PLASTICFOR PRODUCT INFORMATION
1-800-933-0303BAXTER OSMITROL VIAFLEX
AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCBAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USALOT EXP
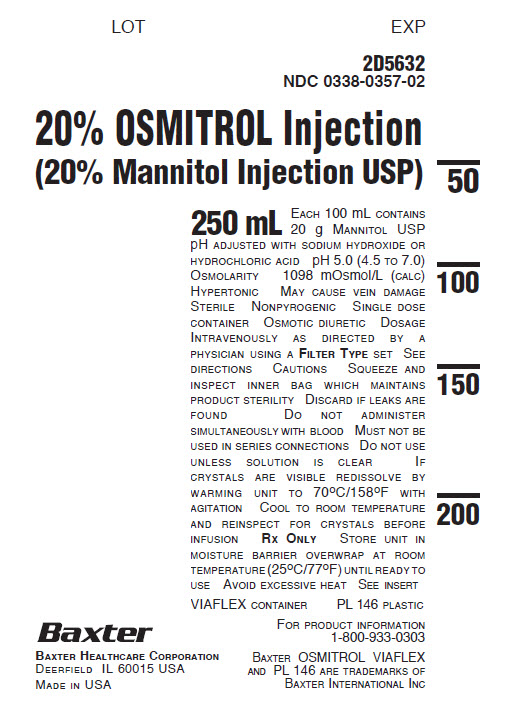
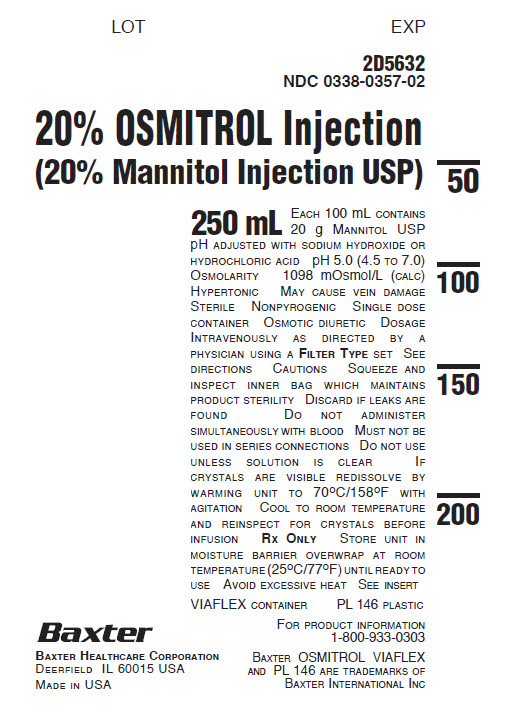
2D5632
NDC 0338-0357-0220% OSMITROL Injection
(20% Mannitol Injection USP)250 mL EACH 100 mL CONTAINS
20 g MANNITOL USP
pH ADJUSTED WITH SODIUM HYDROXIDE OR
HYDROCHLORIC ACID pH 5.0 (4.5 TO 7.0)
OSMOLARITY 1098 mOsmol/L (CALC)
HYPERTONIC MAY CAUSE VEIN DAMAGE
STERILE NONPYROGENIC SINGLE DOSE
CONTAINER OSMOTIC DIURETIC DOSAGE
INTRAVENOUSLY AS DIRECTED BY A
PHYSICIAN USING A FILTER TYPE SET SEE
DIRECTIONS CAUTIONS SQUEEZE AND
INSPECT INNER BAG WHICH MAINTAINS
PRODUCT STERILITY DISCARD IF LEAKS ARE
FOUND DO NOT ADMINISTER
SIMULTANEOUSLY WITH BLOOD MUST NOT BE
USED IN SERIES CONNECTIONS DO NOT USE
UNLESS SOLUTION IS CLEAR IF
CRYSTALS ARE VISIBLE REDISSOLVE BY
WARMING UNIT TO 70°C/158°F WITH
AGITATION COOL TO ROOM TEMPERATURE
AND REINSPECT FOR CRYSTALS BEFORE
INFUSION RX ONLY STORE UNIT IN
MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (25°C/77ºF) UNTIL READY TO
USE AVOID EXCESSIVE HEAT SEE INSERTVIAFLEX CONTAINER PL 146 PLASTIC
FOR PRODUCT INFORMATION
1-800-933-0303BAXTER OSMITROL VIAFLEX
AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCBAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA -
INGREDIENTS AND APPEARANCE
OSMITROL
mannitol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0351 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0351-04 1000 mL in 1 BAG; Type 0: Not a Combination Product 06/08/1964 08/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA013684 06/08/1964 08/31/2019 OSMITROL
mannitol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0353 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0353-03 500 mL in 1 BAG; Type 0: Not a Combination Product 06/08/1964 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA013684 06/08/1964 OSMITROL
mannitol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0355 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 15 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0355-03 500 mL in 1 BAG; Type 0: Not a Combination Product 06/08/1964 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA013684 06/08/1964 OSMITROL
mannitol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0357 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 20 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0357-02 250 mL in 1 BAG; Type 0: Not a Combination Product 06/08/1964 2 NDC:0338-0357-03 500 mL in 1 BAG; Type 0: Not a Combination Product 06/08/1964 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA013684 06/08/1964 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0351, 0338-0353, 0338-0355, 0338-0357) , LABEL(0338-0351, 0338-0353, 0338-0355, 0338-0357) , MANUFACTURE(0338-0351, 0338-0353, 0338-0355, 0338-0357) , PACK(0338-0351, 0338-0353, 0338-0355, 0338-0357) , STERILIZE(0338-0351, 0338-0353, 0338-0355, 0338-0357) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0351, 0338-0353, 0338-0355, 0338-0357)