Label: KOPARI ANTIOXIDANT FACE SHIELD MINERAL SPF 30- zinc oxide lotion
- NDC Code(s): 68577-154-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
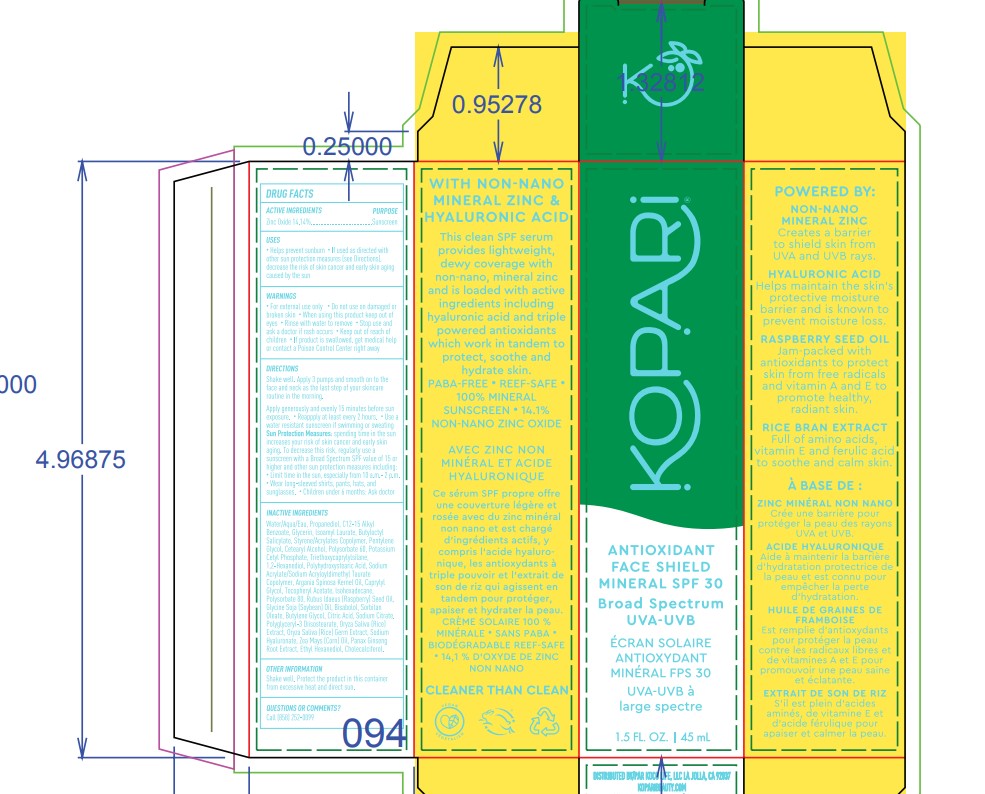
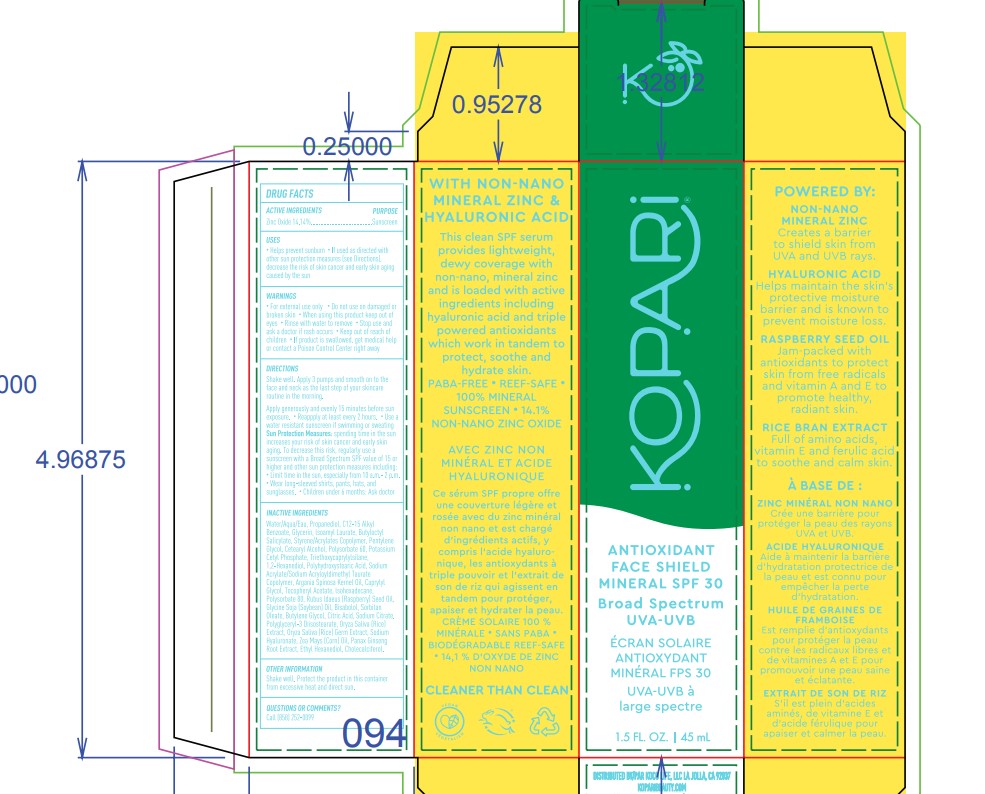
- ACTIVE INGREDIENT
- PURPOSE
- USES
- Warnings
-
DIRECTIONS
Directions
- Shake well. Apply 3 pumps and smooth on to the face and neck as the last step of your skicare routine in the morning.
- Apply generously and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2p.m.
-wear long-sleeved shirts, pants, hats and sunglasses
Children under 6 months of age: Ask a doctor
-
INACTIVE INGREDIENT
Inactive ingredients: water/aqua/eau,propanediol,c12-15 alkyl benzoate,glycerin,lsoamyl laurate,butyloctyl salicylate,styrene/acrylates copolymer,pentylene glycol,cetearyl alcohol,polysorbate 60,potassium cetyl phosphate,sodium hyaluronate,rubus ldaeus (raspberry) seed oil,glycine soja (soybean) oil,oryza sativa (rice) extract, oryza sativa (rice) germ extract, panax ginseng root extract,argania spinosa kernel oil,bisabololzea mays (corn) oil,1,2-hexanediol,butylene glycol,caprylyl glycol,sodium acrylate/sodium acryloyldimethyl taurate copolymer,lsohexadecanepolyhydroxystearic acid,sodium citrate,triethoxycaprylylsilane,polyglyceryl-3 diisostearate,sorbitan oleate,citric acid,tocopheryl acetate,ethyl hexanediol,polysorbate 80,cholecalciferol
- OTHER INFORMATION
- QUESTIONS or COMMENTS
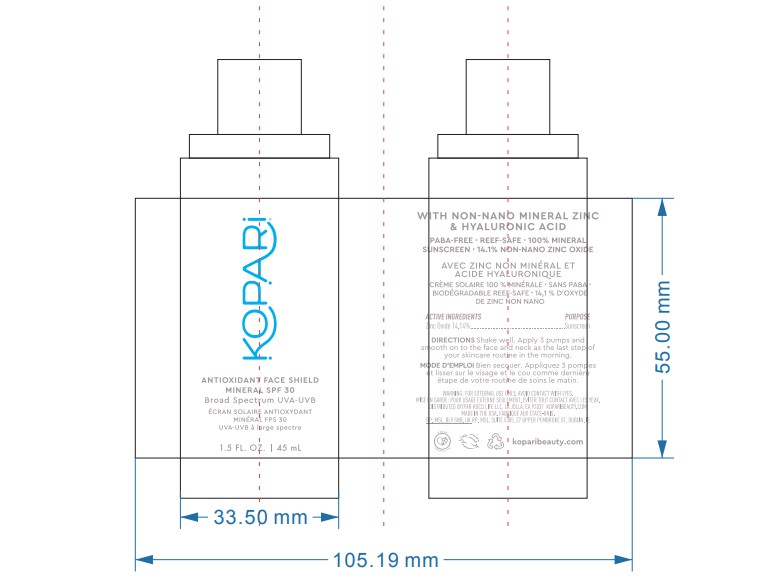
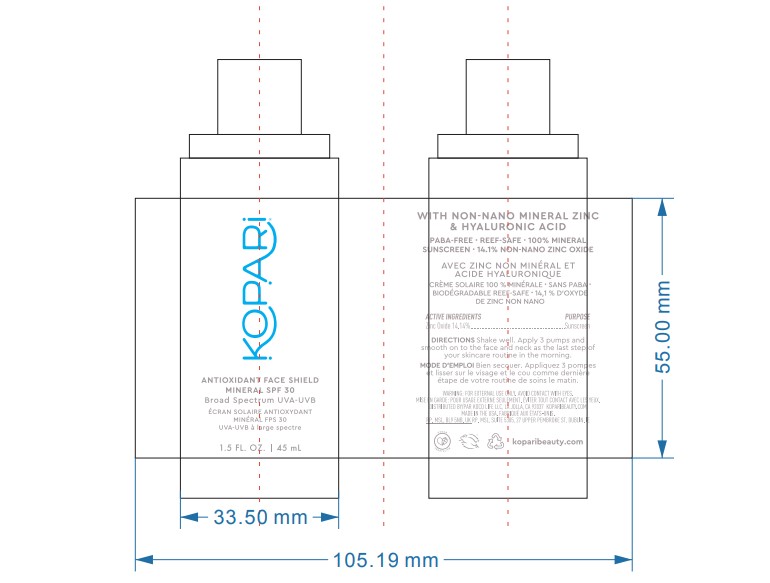
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KOPARI ANTIOXIDANT FACE SHIELD MINERAL SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-154 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.4 mg in 100 mg Inactive Ingredients Ingredient Name Strength CORN OIL (UNII: 8470G57WFM) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ARGAN OIL (UNII: 4V59G5UW9X) WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) ISOAMYL LAURATE (UNII: M1SLX00M3M) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) PENTYLENE GLYCOL (UNII: 50C1307PZG) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) RUBUS IDAEUS SEED (UNII: M3CL7US2ZG) SOYBEAN OIL (UNII: 241ATL177A) ASIAN GINSENG (UNII: CUQ3A77YXI) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ISOHEXADECANE (UNII: 918X1OUF1E) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) LEVOMENOL (UNII: 24WE03BX2T) RICE GERM (UNII: 7N2B70SFEZ) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) CHOLECALCIFEROL (UNII: 1C6V77QF41) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SODIUM CITRATE (UNII: 1Q73Q2JULR) ETHOHEXADIOL (UNII: M9JGK7U88V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-154-01 1 in 1 CARTON 06/01/2023 1 45 mg in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - COSMAX USA, CORPORATION (010990210) Registrant - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-154)