Label: LYFGENIA- lovotibeglogene autotemcel suspension
- NDC Code(s): 73554-1111-1
- Packager: bluebird bio, Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LYFGENIA safely and effectively. See full prescribing information for LYFGENIA.
LYFGENIA® (lovotibeglogene autotemcel) suspension for intravenous infusion
Initial U.S. Approval: 2023WARNING: HEMATOLOGIC MALIGNANCY
See full prescribing information for complete boxed warning.
Hematologic malignancy has occurred in patients treated with LYFGENIA. Monitor patients closely for evidence of malignancy through complete blood counts at least every 6 months and through integration site analysis at Months 6, 12, and as warranted. (5.1)
INDICATIONS AND USAGE
LYFGENIA is an autologous hematopoietic stem cell-based gene therapy indicated for the treatment of patients 12 years of age or older with sickle cell disease and a history of vaso-occlusive events. (1)
Limitations of Use
Following treatment with LYFGENIA, patients with α-thalassemia trait (-α3.7/-α3.7) may experience anemia with erythroid dysplasia that may require chronic red blood cell transfusions. LYFGENIA has not been studied in patients with more than two α-globin gene deletions. (1)
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
- Patients are required to undergo hematopoietic stem cell (HSC) mobilization followed by apheresis to obtain CD34+ cells for LYFGENIA manufacturing. (2.2)
- Dosing of LYFGENIA is based on the number of CD34+ cells in the infusion bag(s) per kg of body weight. (2.1)
- The minimum recommended dose is 3 × 106 CD34+ cells/kg. (2.1)
- Myeloablative conditioning must be administered before infusion of LYFGENIA. (2.2)
- Following myeloablative conditioning, allow a minimum of 48 hours of washout before LYFGENIA infusion. (2.2)
- Verify that the patient's identity matches the unique patient identification information on the LYFGENIA infusion bag(s) prior to infusion. (2.2)
- Do not sample, alter, irradiate, or refreeze LYFGENIA. (2.2)
- Do not use an in-line blood filter or an infusion pump. (2.3)
- Administer LYFGENIA within 4 hours after thawing. (2.3)
- Administer each infusion bag of LYFGENIA via intravenous infusion over a period of less than 30 minutes. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Delayed Platelet Engraftment: Monitor patients frequently for thrombocytopenia and bleeding until platelet engraftment and platelet recovery are achieved. (5.2)
- Neutrophil Engraftment Failure: Monitor absolute neutrophil counts (ANC) after LYFGENIA infusion. If neutrophil engraftment does not occur, administer rescue cells. (5.3)
- Insertional Oncogenesis: There is a potential risk of insertional oncogenesis after treatment with LYFGENIA. (5.4)
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.5)
ADVERSE REACTIONS
Most common adverse reactions ≥ Grade 3 (incidence ≥ 20%) were stomatitis, thrombocytopenia, neutropenia, febrile neutropenia, anemia, and leukopenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact bluebird bio at 1-833-999-6378 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Anti-retrovirals: Discontinue anti-retroviral medications at least one month prior to mobilization and until all cycles of apheresis are completed. There are some long-acting anti-retroviral medications that may require a longer duration of discontinuation for elimination of the medication. (7.2)
- Hydroxyurea: Discontinue 2 months prior to mobilization and 2 days prior to conditioning. (7.3)
- Iron chelation: Discontinue at least 7 days prior to mobilization and conditioning. (7.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEMATOLOGIC MALIGNANCY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation Before LYFGENIA Infusion
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Malignancy
5.2 Delayed Platelet Engraftment
5.3 Neutrophil Engraftment Failure
5.4 Insertional Oncogenesis
5.5 Hypersensitivity Reactions
5.6 Anti-retroviral Use
5.7 Hydroxyurea Use
5.8 Iron Chelation
5.9 Interference with PCR-based Testing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Live Vaccines
7.2 Anti-retrovirals
7.3 Hydroxyurea
7.4 Iron Chelation
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients Seropositive for Human Immunodeficiency Virus (HIV)
8.7 Renal Impairment
8.8 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HEMATOLOGIC MALIGNANCY
Hematologic malignancy has occurred in patients treated with LYFGENIA. Monitor patients closely for evidence of malignancy through complete blood counts at least every 6 months and through integration site analysis at Months 6, 12, and as warranted [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
LYFGENIA is indicated for the treatment of patients 12 years of age or older with sickle cell disease and a history of vaso-occlusive events.
Limitations of Use
Following treatment with LYFGENIA, patients with α-thalassemia trait (-α3.7/-α3.7) may experience anemia with erythroid dysplasia that may require chronic red blood cell transfusions [see Adverse Reactions (6.1)]. LYFGENIA has not been studied in patients with more than two α-globin gene deletions.
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For one-time single-dose intravenous use only.
2.1 Dose
LYFGENIA is provided as a single dose for infusion containing a suspension of CD34+ cells in one to four infusion bags. The minimum recommended dose of LYFGENIA is 3 × 106 CD34+ cells/kg.
See the Lot Information Sheet provided with the product shipment for additional information pertaining to dose.
2.2 Preparation Before LYFGENIA Infusion
Confirm that autologous hematopoietic stem cell (HSC) transplantation is appropriate for the patient before mobilization and apheresis and before myeloablative conditioning are initiated. Perform screening for infectious diseases, specifically human immunodeficiency virus 1 & 2 (HIV-1/HIV-2), in accordance with clinical guidelines before collection of cells for manufacturing. There are no data on use of LYFGENIA in HIV-positive patients.
Prepare for Mobilization and Apheresis
Prepare patients for mobilization with at least 2 cycles of scheduled transfusions (one each month) with erythrocytapheresis being preferred. For at least 60 days prior to mobilization and through myeloablative conditioning, patients should undergo a transfusion regimen to reach a target Hb of 8-10 g/dL, not to exceed 12 g/dL, and HbS of less than 30% to reduce the risk of SCD-related complications. Perform erythrocytapheresis within a recommended 4 days preceding mobilization to reach the target of less than 30% HbS.
Manage other concomitant medications (as applicable) as described below:
- Hydroxyurea: Discontinue at least 2 months prior to mobilization. Patients should not resume hydroxyurea until all cycles of apheresis are completed.
- Disease-modifying agents (e.g., L-glutamine, voxelotor and crizanlizumab): Discontinue at least 2 months prior to mobilization as the interaction between disease modifying agents and mobilization agents is unknown.
- Erythropoietin: Discontinue at least 2 months prior to mobilization.
- Iron chelation: Discontinue at least 7 days prior to mobilization.
- Granulocyte-colony stimulating factor (G-CSF): Do not administer G-CSF prior to or with mobilization agents.
- Anti-retrovirals: Discontinue prophylactic HIV anti-retroviral medications at least one month prior to mobilization and do not resume until all cycles of apheresis are completed. There are some long-acting anti-retroviral medications that may require a longer duration of discontinuation for elimination of the medication.
Mobilization and Apheresis
Perform HSC mobilization followed by apheresis to obtain CD34+ cells for product manufacturing. Administer plerixafor to mobilize stem cells prior to the apheresis procedure at a dose of 0.24 mg/kg/day.1
Begin apheresis approximately 4 to 6 hours after plerixafor administration. If more than one apheresis day is required, confirm platelet counts to be ≥ 75 × 109/L within 24 hours of subsequent apheresis sessions, prior to administration of plerixafor on that day. If platelet counts do not meet these criteria, defer mobilization and apheresis until the platelet counts recover to ≥ 75 × 109/L. For patients undergoing more than 1 mobilization cycle, separate each cycle by at least 14 days. Administer daily plerixafor 4 to 6 hours prior to each apheresis collection. If a sufficient number of cells are collected after the first mobilization cycle, no further mobilization/apheresis is required. In clinical studies, the minimum number of CD34+ cells to manufacture LYFGENIA was collected in most patients with 1 or 2 cycles of mobilization and apheresis which typically required 2 consecutive collection days per cycle.
Maximize CD34+ cell collection to obtain as many CD34+ stem cells as possible for product manufacturing during each mobilization and apheresis cycle. Target a minimum collection of 16.5 × 106 CD34+ cells/kg for manufacturing and back-up. If, after manufacturing, the minimum dose of 3 × 106 CD34+ cells/kg is not achieved, the patient may undergo additional cycles of mobilization and apheresis, separated by at least 14 days, to obtain more cells for additional manufacture. Multiple drug product lots may be administered to comprise the final dose.
Retain ≥ 1.5 × 106 CD34+ cells/kg from the collection for back-up. These cells must be collected from the patient and be cryopreserved prior to myeloablative conditioning. The back-up collection may be needed for rescue treatment if there is: 1) compromise of hematopoietic stem cells or LYFGENIA before infusion, 2) primary engraftment failure, or 3) loss of engraftment after infusion with LYFGENIA.
Manage concomitant medications (as applicable) between apheresis and conditioning as described below:
- Transfusions: Scheduled transfusions can be continued between apheresis and conditioning. Patients should maintain total hemoglobin (Hb) levels of 8 to 10 g/dL. Hb levels should not exceed 12 g/dL. A scheduled transfusion should be performed within 2 days prior to conditioning.
- Hydroxyurea: If administered after apheresis, discontinue hydroxyurea at least 2 days prior to myeloablative conditioning.
- Disease-modifying agents (e.g., L-glutamine, voxelotor and crizanlizumab): Discontinue disease modifying agents at least 2 months prior to conditioning as the interaction between disease modifying agents and conditioning agents are unknown.
- Iron chelation: If administered after apheresis, discontinue iron chelation at least 7 days prior to myeloablative conditioning.
- G-CSF: Do not administer G-CSF between mobilization and conditioning.
- Anti-retrovirals and erythropoietin: There are no data regarding use of anti-retrovirals or erythropoietin between apheresis and conditioning.
Myeloablative Conditioning
Do not begin myeloablative conditioning until the complete set of infusion bag(s) constituting the dose of LYFGENIA has been received and stored at the treatment center and the availability of the back-up collection is confirmed.
Myeloablative conditioning with busulfan must be administered before infusion of LYFGENIA.
Busulfan should be administered via a central venous line. For patients weighing less than 35 kg, administer every 6 hours; for patients weighing 35 kg or more, busulfan can be administered either once daily or every 6 hours. Calculate the dose on the basis of the lower of the ideal versus actual body weight.
The recommended initial dose of busulfan is 3.2 mg/kg/day for 4 consecutive days as a 3-hour infusion for a total of 4 doses.2 To achieve myeloablation, the busulfan target AUC is 5000 (range 4400 to 5400) μM*min for a once daily dosing regimen.
For divided dosing, initial dose of busulfan is 0.8 mg/kg/dose every 6 hours as a 2-hour infusion for 16 consecutive doses. Target AUC is 1250 (range 1100 to 1350) μM*min for a q6h dosing regimen.
Perform pharmacokinetic (PK) monitoring after the first dose and adjust dose to achieve the target AUC. Adjust sample collection based on whether a 2-hour or 3-hour infusion is used. If feasible, daily busulfan PK measurement is recommended.
Administer seizure prophylaxis with agents other than phenytoin at least 12 hours prior to initiating busulfan. Do not use phenytoin because of its induction of cytochrome P450 and resultant increased clearance of busulfan.
Consider prophylaxis for hepatic veno-occlusive disease (VOD)/hepatic sinusoidal obstruction syndrome with ursodeoxycholic acid or defibrotide.
After completion of the myeloablative conditioning, allow a minimum of 48 hours of washout before LYFGENIA infusion.
Receipt and Storage of LYFGENIA
- LYFGENIA is shipped in the vapor phase of liquid nitrogen shipper.
- Confirm patient identifiers on the product label(s) and Lot Information Sheet within the shipper.
- If there are any concerns about the product or packaging upon receipt, contact bluebird bio at 1-833-999-6378.
- Keep the infusion bag(s) in the metal cassette(s) and transfer LYFGENIA from the vapor phase of liquid nitrogen shipper to the treatment center vapor phase of liquid nitrogen storage at ≤ -140°C (-220°F). Store in the vapor phase of liquid nitrogen at ≤ -140°C (-220°F) until ready for thaw and administration.
Preparation of LYFGENIA for Infusion
Coordinate the timing of LYFGENIA thaw and infusion. Confirm the infusion time in advance and adjust the start time of LYFGENIA thaw such that it will be available for infusion when the patient and healthcare providers are ready. Note that each infusion bag must be completely administered within 4 hours after thawing.
- Remove each metal cassette from liquid nitrogen storage and remove each infusion bag from the metal cassette.
- Confirm that LYFGENIA is printed on the infusion bag(s).
- Confirm that patient identity matches the unique patient identifiers located on the LYFGENIA infusion bag(s). Do not infuse LYFGENIA if the information on the patient-specific label on the infusion bag does not match the intended patient and contact bluebird bio at 1-833-999-6378.
- Ensure the correct number of infusion bags are present. Use the accompanying Lot Information Sheet to confirm that each infusion bag is within the expiration date.
- Inspect each infusion bag for any breaches of integrity before and after thawing and before infusion. If an infusion bag is compromised, follow the local guidelines and contact bluebird bio immediately at 1-833-999-6378.
- If more than one infusion bag is provided, thaw and administer each infusion bag completely before proceeding to thaw the next infusion bag. Maintain any additional infusion bag(s), if applicable, at less than or equal to -140°C (-220°F) until time to thaw.
- Thaw LYFGENIA at 37°C (98.6°F) in a water bath or dry bath. Thawing of each infusion bag takes approximately 2 to 4 minutes. Do not leave LYFGENIA unattended. Do not submerge the infusion ports in a water bath.
- After thaw, mix the contents gently by massaging the infusion bag to disperse clumps of cellular material until all of the contents are uniform. If visible cell clumps remain, continue to gently mix the contents of the bag. Most small clumps of cellular material should disperse with gentle manual mixing. Do not filter, wash, spin down and/or resuspend LYFGENIA in new media prior to infusion.
- Do not sample, alter, irradiate or refreeze LYFGENIA.
2.3 Administration
LYFGENIA is for autologous use only. The patient's identity must match the patient identifiers on the LYFGENIA cassette(s) and infusion bag(s). Do not infuse LYFGENIA if the information on the patient-specific label does not match the intended patient.
- Administer LYFGENIA within 4 hours after thawing.
- Do not use an in-line blood filter or an infusion pump.
- Before infusion, confirm that the patient's identity matches the unique patient identifiers on the LYFGENIA infusion bag(s). Use the Lot Information Sheet to confirm the total number of infusion bags to be administered.
- Expose the sterile port on the infusion bag by tearing off the protective wrap covering the port.
- Infuse LYFGENIA as soon as possible after thawing and complete the infusion within 4 hours.
- Administer each infusion bag of LYFGENIA via intravenous infusion over a period of less than 30 minutes. If more than one infusion bag is provided, administer the contents of each infusion bag completely before proceeding to thaw and infuse the contents of the next infusion bag.
- Administer the entire contents of each infusion bag to ensure that as many cells as possible are infused into the patient. After administration of each drug product, the infusion bag and any associated tubing are flushed with at least 50 mL of 0.9% sodium chloride solution to ensure as many cells as possible are infused into the patient.
After LYFGENIA Administration
Standard procedures for patient management after HSC transplantation should be followed after LYFGENIA infusion.
- Irradiate any blood products required for at least the first 3 months after LYFGENIA infusion and per transplant physician's recommendation.
- There is no experience regarding the use of hydroxyurea, anti-retrovirals, erythropoietin or disease-modifying agents, such as voxelotor or crizanlizumab, after LYFGENIA infusion.
- Avoid use of myelosuppressive iron chelators for 6 months. If iron chelation is needed, consider administration of non-myelosuppressive iron chelators. Phlebotomy can be used in lieu of iron chelation when appropriate.
- G-CSF is not recommended for 21 days after LYFGENIA infusion.
- Patients should not donate blood, organs, tissues, or cells at any time in the future.
LYFGENIA contains human blood stem cells that are genetically modified with a replication-incompetent, self-inactivating lentiviral vector (LVV). Follow universal precautions and local biosafety guidelines (Biosafety Level 2) for handling and disposal of LYFGENIA to avoid potential transmission of infectious diseases.
-
3 DOSAGE FORMS AND STRENGTHS
LYFGENIA is a cell suspension for intravenous infusion.
LYFGENIA is composed of one to four infusion bags which contain 1.7 to 20 × 106 cells/mL suspended in cryopreservation solution [see How Supplied/Storage and Handling (16)]. Each infusion bag contains approximately 20 mL of LYFGENIA. A single dose of LYFGENIA contains a minimum of 3 × 106 CD34+ cells per kg of body weight, suspended in cryopreservation solution.
See the Lot Information Sheet for actual dose.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic Malignancy
Hematologic malignancy has occurred in patients treated with LYFGENIA (Study 1, Group A). At the time of initial product approval, two patients treated with an earlier version of LYFGENIA using a different manufacturing process and transplant procedure (Study 1, Group A) developed acute myeloid leukemia (AML). One patient with α-thalassemia trait (Study 1, Group C) has been diagnosed with myelodysplastic syndrome (MDS) [see Adverse Reactions (6.1)].
The additional hematopoietic stress associated with mobilization, conditioning, and infusion of LYFGENIA, including the need to regenerate the hematopoietic system, may increase the risk of a hematologic malignancy.
Patients with sickle cell disease have an increased risk of hematologic malignancy as compared to the general population.3, 4 Patients treated with LYFGENIA may develop hematologic malignancies and should have lifelong monitoring. Monitor for hematologic malignancies with a complete blood count (with differential) at least every 6 months for at least 15 years after treatment with LYFGENIA, and integration site analysis at Months 6, 12, and as warranted.
In the event that a malignancy occurs, contact bluebird bio at 1-833-999-6378 for reporting and to obtain instructions on collection of samples for testing.
Post-Marketing Long Term Follow-Up Study
Patients who intend to receive treatment with LYFGENIA are encouraged to enroll in the study, as available, to assess the long-term safety of LYFGENIA and the risk of malignancies occurring after treatment with LYFGENIA by calling bluebird bio at 1-833-999-6378. The study includes monitoring (at pre-specified intervals) for clonal expansion.
5.2 Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with LYFGENIA. Bleeding risk is increased prior to platelet engraftment and may continue after engraftment in patients with prolonged thrombocytopenia. Two patients (4%) required more than 100 days post treatment with LYFGENIA to achieve platelet engraftment [see Adverse Reactions (6.1)].
Patients should be made aware of the risk of bleeding until platelet recovery has been achieved. Monitor patients for thrombocytopenia and bleeding according to standard guidelines. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
5.3 Neutrophil Engraftment Failure
There is a potential risk of neutrophil engraftment failure after treatment with LYFGENIA. Neutrophil engraftment failure is defined as failure to achieve three consecutive absolute neutrophil counts (ANC) ≥ 0.5 × 109 cells/L obtained on different days by Day 43 after infusion of LYFGENIA. Monitor neutrophil counts until engraftment has been achieved. If neutrophil engraftment failure occurs in a patient treated with LYFGENIA, provide rescue treatment with the back-up collection of CD34+ cells [see Adverse Reactions (6.1)].
5.4 Insertional Oncogenesis
There is a potential risk of lentiviral vector-mediated insertional oncogenesis after treatment with LYFGENIA.
5.5 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of LYFGENIA. The dimethyl sulfoxide (DMSO) or dextran 40 in LYFGENIA may cause hypersensitivity reactions, including anaphylaxis.
5.6 Anti-retroviral Use
Patients should not take prophylactic HIV anti-retroviral medications for at least one month prior to mobilization and until all cycles of apheresis are completed. There are some long-acting anti-retroviral medications that may require a longer duration of discontinuation for elimination of the medication [see Dosing and Administration (2.2) and Drug Interactions (7.2)].
If a patient is taking anti-retrovirals for HIV prophylaxis, confirm a negative test for HIV before beginning mobilization and apheresis of CD34+ cells.
5.7 Hydroxyurea Use
Patients should not take hydroxyurea for at least 2 months prior to mobilization and until all cycles of apheresis are completed. If hydroxyurea is administered between mobilization and conditioning, discontinue 2 days prior to initiation of conditioning [see Dosing and Administration (2.2) and Drug Interactions (7.3)].
5.8 Iron Chelation
Do not administer myelosuppressive iron chelators for 6 months post-treatment with LYFGENIA[see Dosing and Administration (2.2) and Drug Interactions (7.4)].
5.9 Interference with PCR-based Testing
Patients who have received LYFGENIA are likely to test positive by polymerase chain reaction (PCR) assays for HIV due to integrated BB305 LVV proviral DNA, resulting in a possible false-positive PCR assay test result for HIV. Therefore, patients who have received LYFGENIA should not be screened for HIV infection using a PCR-based assay.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Hematologic Malignancy [see Warnings and Precautions (5.1)]
- Delayed Platelet Engraftment [see Warnings and Precautions (5.2)]
- Neutrophil Engraftment Failure [see Warnings and Precautions (5.3)]
- Insertional Oncogenesis [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety was based on patients with sickle cell disease in one open-label, single-arm clinical trial and one long-term follow-up study. Of the 54 patients who initiated stem cell collection, the median (min, max) age across the studies was 25 (12, 43) years, 63% were males, 89% were Black or African American, 2% were Asian, 2% White/Caucasian and 4% were not reported. The median (min, max) duration of follow-up was 42 (12, 87) months.
Mobilization and apheresis triggered SAEs of sickle cell crisis in 6 (14%, 6/44) patients who initiated mobilization in the intent-to-treat population.
All patients who initiated conditioning (100%, 45/45) experienced at least one adverse event attributed to conditioning. The majority of conditioning-attributed events were non-serious and were consistent with the known effects of alkylating agents.
Thirty-three (73%, 33/45) patients who received LYFGENIA experienced at least one serious adverse event (SAE). Most SAEs were related to conditioning or underlying disease.
Table 1 presents the adverse drug reactions following treatment with LYFGENIA (Day 1) to Month 24.
Table 1: Adverse Reactions ≥ Grade 3 (> 5%) Following Treatment with LYFGENIA from Day 1 to Month 24 (N = 45)* Adverse Reaction Grade 3 or Higher
n (%)Blood and lymphatic system disorders -- Thrombocytopenia 31 (69) Neutropenia 27 (60) Febrile neutropenia 20 (44) Anemia† 15 (33) Leukopenia 15 (33) Sickle cell anemia with crisis‡ 7 (16) Gastrointestinal disorders -- Stomatitis 32 (71) Nausea 4 (9) General disorders and administration site conditions -- Pyrexia 3 (7) Infections and infestations -- Bacteremia 3 (7) Investigations -- Aspartate aminotransferase increased 8 (18) Alanine aminotransferase increased 6 (13) Gamma-glutamyl transferase increased 6 (13) Blood bilirubin increased 3 (7) Metabolism and nutrition disorders -- Decreased appetite 5 (11) Respiratory, thoracic, and mediastinal disorders -- Pharyngeal inflammation 5 (11) Three patients died during LYFGENIA clinical trials; one from sudden cardiac death due to underlying disease and two from acute myeloid leukemia who were treated with an earlier version of LYFGENIA using a different manufacturing process and transplant procedure (Study 1, Group A).
Anemia
Two patients developed anemia following LYFGENIA treatment; one patient continues to require monthly packed red blood cell (pRBC) transfusions. The other patient has been diagnosed with MDS. Both subjects had α-thalassemia trait (-α3.7 /-α3.7) [see Limitations of Use (1)].
Infusion-related reactions to LYFGENIA
Pre-medication for infusion reactions was managed at physician discretion. Infusion-related reactions to LYFGENIA were observed in 2 patients on the day of LYFGENIA infusion. Both infusion-related reactions resolved and were Grade 1. Events included hot flush and decreased diastolic blood pressure.
Platelet engraftment delay
Platelet engraftment, defined as having 3 consecutive platelet values ≥ 50 × 109/L obtained on different days after the initial post-transplant nadir without receiving any platelet transfusions for 7 days immediately preceding and during the evaluation period, was achieved in all patients (median [min, max] Day 37 [19, 235]) after LYFGENIA infusion. Two patients treated with LYFGENIA achieved platelet engraftment after Day 100; one of these patients was administered eltrombopag until Day 234 [see Warnings and Precautions (5.2)].
-
7 DRUG INTERACTIONS
No formal drug interaction studies have been performed. LYFGENIA is not expected to interact with the hepatic cytochrome P-450 family of enzymes or drug transporters.
7.1 Live Vaccines
Follow institutional guidelines for vaccine administration. The safety of immunization with live viral vaccines during or following LYFGENIA treatment has not been studied. Recommendations for vaccination schedules should be followed as per guidelines post-autologous hematopoietic stem cell transplant and functional asplenia.
7.2 Anti-retrovirals
Patients should not take anti-retroviral medications for at least one month prior to mobilization for required and until all cycles of apheresis are completed [see Warnings and Precautions (5.6)]. There are some long-acting anti-retroviral medications that may require a longer duration of discontinuation for elimination of the medication. Anti-retroviral medications may interfere with manufacturing of LYFGENIA.
7.3 Hydroxyurea
Patients should not take hydroxyurea for at least 2 months prior to mobilization and until all cycles of apheresis are completed and should discontinue 2 days prior to initiation of conditioning [see Warnings and Precautions (5.7)].
7.4 Iron Chelation
Drug-drug interactions between iron chelators and the mobilization process and myeloablative conditioning agent must be considered. Iron chelators should be discontinued at least 7 days prior to initiation of mobilization or conditioning. Myelosuppressive iron chelators (e.g., deferiprone) should be restarted no sooner than 6 months after LYFGENIA infusion [see Warnings and Precautions (5.8)]. Non-myelosuppressive iron chelation should be restarted no sooner than 3 months after LYFGENIA infusion. Phlebotomy can be used in lieu of iron chelation, when appropriate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on LYFGENIA administration in pregnant women. Consider the risks associated with myeloablative conditioning agents on pregnancy and fertility.
No reproductive and developmental toxicity studies in animals have been conducted with LYFGENIA to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known whether LYFGENIA has the potential to be transferred to the fetus. Therefore, LYFGENIA should not be administered to women who are pregnant, and pregnancy after LYFGENIA infusion should be discussed with the treating physician.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of LYFGENIA in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for LYFGENIA and any potential adverse effects on the breastfed child from LYFGENIA. Therefore, LYFGENIA is not recommended for women who are breastfeeding, and breastfeeding after LYFGENIA infusion should be discussed with the treating physician.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
A negative serum pregnancy test must be confirmed prior to the start of mobilization and re‑confirmed prior to conditioning procedures and before LYFGENIA administration.
Contraception
There are insufficient exposure data to provide a precise recommendation on duration of contraception following treatment with LYFGENIA.
Women of childbearing potential and men capable of fathering a child should use an effective method of contraception (intra-uterine device or combination of hormonal and barrier contraception) from start of mobilization through at least 6 months after administration of LYFGENIA. Advise patients of the risks associated with conditioning agents.
8.4 Pediatric Use
The safety and efficacy of LYFGENIA have been established in pediatric patients 12 years of age and older with sickle cell disease, including 8 adolescents (age 12 years to less than 18) [see Clinical Studies (14)].
No clinically meaningful differences in efficacy or safety were observed between the adult and pediatric subgroups.
The safety and efficacy of LYFGENIA in children less than 12 years of age have not been established. No data are available.
8.5 Geriatric Use
LYFGENIA has not been studied in patients 65 years of age and older. Autologous hematopoietic stem cell (HSC) transplantation must be appropriate for a patient to be treated with LYFGENIA.
8.6 Patients Seropositive for Human Immunodeficiency Virus (HIV)
LYFGENIA has not been studied in patients with HIV-1 or HIV-2. A negative serology test for HIV is necessary prior to apheresis. Patients with a positive test for HIV will not be accepted for LYFGENIA treatment.
-
11 DESCRIPTION
LYFGENIA (lovotibeglogene autotemcel) is a βA-T87Q-globin gene therapy consisting of autologous CD34+ cells from patients with sickle cell disease containing hematopoietic stem cells (HSCs) transduced with BB305 LVV encoding βA-T87Q-globin, suspended in cryopreservation solution. LYFGENIA is intended for one-time administration to add functional copies of a modified form of the β-globin gene (βA-T87Q-globin gene) into the patient's own HSCs.
LYFGENIA is prepared using the patient's own HSCs, which are collected via apheresis procedure(s). The autologous cells are enriched for CD34+ cells, then transduced ex vivo with BB305 LVV. The promoter, a regulatory element that controls the expression of the transgene selected for BB305 LVV, is a cellular (non-viral) promoter that controls gene expression specific to the erythroid lineage cells (red blood cells and their precursors). BB305 LVV encodes βA-T87Q-globin.
The transduced CD34+ cells are washed, formulated into a suspension, and then cryopreserved. LYFGENIA is frozen in a patient-specific infusion bag(s) and is thawed prior to administration [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)]. The thawed product is colorless to white to red, including shades of white or pink, light yellow, and orange, and may contain small proteinaceous particles. Due to the presence of cells, the solution may be clear to slightly cloudy and may contain visible cell aggregates.
The formulation contains 5% dimethyl sulfoxide (DMSO).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
LYFGENIA adds functional copies of a modified βA-globin gene (threonine [T] replaced with glutamine [Q] at position 87, T87Q or βA-T87Q-globin) into patients' hematopoietic stem cells (HSCs) through transduction of autologous CD34+ cells with BB305 LVV. After LYFGENIA infusion, the transduced CD34+ HSCs engraft in the bone marrow and differentiate to produce red blood cells containing biologically active βA-T87Q-globin that will combine with α-globin to produce functional Hb containing βA-T87Q-globin (HbAT87Q). βA-T87Q-globin can be distinguished from wildtype βA-globin and from βS-globin through reverse-phase high-performance liquid chromatography (RPHPLC) or ultra-high performance liquid chromatography (UPLC). HbAT87Q has similar oxygen-binding affinity and oxygen hemoglobin dissociation curve to wild type HbA, reduces intracellular and total hemoglobin S (HbS) levels, and is designed to sterically inhibit polymerization of HbS thereby limiting the sickling of red blood cells.
12.2 Pharmacodynamics
HbAT87Q generally increased steadily after LYFGENIA infusion and stabilized by approximately Month 6 after infusion. Patients had a Month 6 median (min, max) HbAT87Q of 5.2 (2.6, 8.8) g/dL in an ongoing Phase 1/2 Study Group C (Study 1-C) (N = 33). HbAT87Q remained durable with a median (min, max) of 5.5 (2.4, 9.4) g/dL at Month 24 (N = 34). HbAT87Q comprised a median (min, max) 45.7 (26.9, 63.2) (N = 34) percent of total non-transfused Hb at Month 24.
Expression of HbAT87Q continued to remain durable through Month 48 (N = 10), demonstrating sustained expression of the βA-T87Q protein derived from irreversible integration of the βA-T87Q-globin gene into long-term hematopoietic stem cells (HSCs).
12.3 Pharmacokinetics
LYFGENIA is an autologous gene therapy which includes hematopoietic stem cells (HSCs) that have been genetically modified ex vivo. The nature of LYFGENIA is such that conventional studies on pharmacokinetics, absorption, distribution, metabolism, and elimination are not applicable.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of LYFGENIA was studied in a single-arm, 24-month, open-label, multicenter Phase 1/2 study (Study 1-C) and continued on a long-term follow-up study. In Study 1-C, 43 subjects underwent apheresis after mobilization with plerixafor of which 36 patients received myeloablative busulfan conditioning. Seven patients did not proceed to conditioning; 2 patients discontinued due to apheresis-related issues and 5 discontinued at patient and/or physician discretion.
Thirty-six patients received the intravenous infusion of LYFGENIA with a median (min, max) dose of 6.4 (3, 14) × 106 CD34+ cells/kg (48 hours after the last dose of busulfan). (See Section 2 for Dosage and Administration for Mobilization and Apheresis and Myeloablative Conditioning.)
As LYFGENIA is an autologous therapy, prophylactic long-term immunosuppressive agents were not required in clinical studies. No patients experienced graft failure or graft rejection. (See Section 6 for details of neutrophil and platelet engraftment.)
Table 2 includes the demographics and baseline characteristics for patients in Study 1-C.
Table 2: Demographics and Characteristics for Patients Treated with LYFGENIA in Study 1-C Transplant Population Transplant Population for VOE Efficacy Outcomes Attribute N = 36 N = 32 - *
- Patients with a history of stroke were included in early inclusion criteria in Study 1-C.
β-globin Genotype: βS/βS, n (%) 36 (100) 32 (100) α-globin Genotype: αα/αα, n (%) 23 (64) 20 (63) α-globin Genotype: αα/-α3.7, n (%) 11 (31) 10 (31) α-globin Genotype: -α3.7/-α3.7, n (%) 2 (6) 2 (6) Age in years, median (min, max) 24 (12, 38) 25 (12, 38) Age in years, n (%) ≥ 18 years 28 (78) 24 (75) ≥ 12 years to < 18 8 (22) 8 (25) Sex: Male, n (%) 22 (61) 20 (63) Race: Black/African American n (%) 35 (97) 31 (97) Race: Not Reported n (%) 1 (3) 1 (3) Patients with History of Stroke*, n (%) 5 (14) 1 (3) Efficacy Outcomes
The transplant population for VOE efficacy outcomes included patients with a history of at least 4 VOEs in the 24 months prior to informed consent. The efficacy outcomes were complete resolution of VOEs (VOE-CR) and severe VOEs (sVOE-CR) between 6 months and 18 months after infusion of LYFGENIA.
VOEs were defined as any of the following events requiring evaluation at a medical facility:
- an episode of acute pain with no medically determined cause other than vaso-occlusion, lasting more than 2 hours
- acute chest syndrome (ACS)
- acute hepatic sequestration
- acute splenic sequestration
Severe VOE (sVOE) were defined as either of the following events:
- VOE requiring a hospitalization or multiple visits to an emergency department/urgent care over 72 hours and receiving intravenous medications at each visit
- priapism requiring any level of medical attention
Table 3: Summary of VOE Efficacy Outcomes for Patients in Study 1-C Clinical Attribute Results (s)VOE-CR = elimination of (s)VOEs between 6 and 18 months post infusion with LYFGENIA. VOE-CR n/N (%) 28/32 (88%) [95% CI] [71, 97] sVOE-CR n/N (%) 30/32 (94%) [95% CI] [79, 99] Globin response (GR) was defined as meeting the following criteria for a continuous period of at least 6 months after drug product infusion:
- weighted average hemoglobin AT87Q percentage of non-transfused total Hb ≥ 30% AND
- weighted average non-transfused total Hb (HbS+HbF+HbA2+HbAT87Q) increase of ≥ 3 g/dL compared to baseline total Hb OR weighted average non-transfused total Hb ≥ 10 g/dL.
All 36 patients infused in Study 1-C (transplant population) were evaluated for globin response. 31/36 (86%) achieved GR. All patients maintained GR once it was achieved.
The median (min, max) duration of follow-up for the patients in Study 1-C (N = 36) is 38 (12, 61) months post LYFGENIA infusion. After the primary evaluation period to last follow-up, 4 of 32 patients who achieved VOE-CR experienced VOEs while maintaining GR. After the primary evaluation period up to 24 months, 17 of 35 (49%) patients were prescribed opioids for sickle cell and non-sickle cell-related pain.
-
15 REFERENCES
- 1
- Tisdale JF, Pierciey FJ, Bonner M, et al. (2020) Safety and feasibility of hematopoietic progenitor stem cell collection by mobilization with plerixafor followed by apheresis vs bone marrow harvest in patients with sickle cell disease in the multi-center HGB-206 trial. Am J Hematol E239–E242. https://doi.org/10.1002/ajh.25867.
- 2
- Palmer J, McCune JS, Perales M-A, et al. (2016) Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. Biol Blood Marrow Transplant 1915–1925. https://doi.org/10.1016/j.bbmt.2016.07.013
- 3
- Brunson A, Keegan THM, Bang H, et al. (2017) Increased risk of leukemia among sickle cell disease patients in California. Blood 130:1597–1599. doi: 10.1182/blood-2017-05-783233.
- 4
- Seminog OO, Ogunlaja OI, Yeates D, Goldacre MJ (2016) Risk of individual malignant neoplasms in patients with sickle cell disease: English national record linkage study. J R Soc Med 109:303–309. doi: 10.1177/0141076816651037.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
LYFGENIA is supplied in one to four infusion bags containing a frozen suspension of genetically modified autologous cells, enriched for CD34+ cells. Each bag contains approximately 20 mL. Each infusion bag is individually packed within an overwrap in a metal cassette. LYFGENIA is shipped from the manufacturing facility to the treatment center storage facility in a cryoshipper, which may contain multiple metal cassettes intended for a single patient. A Lot Information Sheet is affixed inside the shipper.
- 20 mL infusion bag, overwrap, and metal cassette (NDC 73554-1111-1)
Match the identity of the patient with the patient identifiers on the metal cassette(s), infusion bag(s), and Lot Information Sheet upon receipt.
- Keep the infusion bag(s) in the metal cassette(s) and store in the vapor phase of liquid nitrogen at less than or equal to -140°C (≤ -220°F) until ready for thaw and administration.
- Thaw LYFGENIA prior to infusion [see Dosage and Administration (2.2)].
- Do not re-freeze after thawing.
- Do not irradiate LYFGENIA, as this could lead to inactivation.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure. In case of manufacturing failure or the need for additional cells, additional cell collection and manufacturing of LYFGENIA would be needed [see Dosage and Administration (2.2)].
Prior to treatment, advise patients of the following:
- Risks associated with mobilization and myeloablative conditioning agents [see Use in Specific Populations (8.1, 8.3)].
- Hematologic Malignancy – Hematologic malignancy has occurred in patients treated with LYFGENIA. Patients with sickle cell disease have an increased risk of hematologic malignancy as compared to the general population. The additional hematopoietic stress associated with mobilization, conditioning, and infusion of LYFGENIA, including the need to regenerate the hematopoietic system, may increase the risk of a hematologic malignancy [see Warnings and Precautions (5.1)].
- Delayed Platelet Engraftment - Delayed platelet engraftment has been observed with LYFGENIA. Patients should be made aware of the risk of bleeding until platelet recovery has been achieved [see Warnings and Precautions (5.2)].
- Risk of Neutrophil Engraftment Failure – Patients who experience neutrophil engraftment failure will receive rescue treatment with their back-up collection of CD34+ cells [see Warnings and Precautions (5.3)].
- Insertional Oncogenesis – There is a potential risk of insertional oncogenesis after treatment with LYFGENIA [see Warnings and Precautions (5.4)].
- Patients should be monitored lifelong. Monitoring will include assessment for hematologic malignancies with a complete blood count at least every 6 months for at least 15 years after treatment with LYFGENIA. This will include integration site analysis at Months 6, 12, and as warranted [see Warnings and Precautions (5.1) and (5.4)].
Advise patients:
- to have their treating physician contact bluebird bio at 1-833-999-6378 if they are diagnosed with a malignancy [see Warnings and Precautions (5.1, 5.4)].
- to monitor for signs and symptoms of bleeding and have frequent blood draws for platelet counts, until platelet recovery has been achieved [see Warnings and Precautions (5.2)].
- that they may test positive for HIV if tested using a PCR assay after being treated with LYFGENIA [see Warnings and Precautions (5.9)].
- that they should not donate blood, organs, tissues, or cells at any time in the future [see Dosage and Administration (2.3)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
LYFGENIA® (pronounced lif-JEN-ee-uh)
(lovotibeglogene autotemcel)This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: December 2023What is the most important information I should know about LYFGENIA?
Patients treated with LYFGENIA have developed blood cancers. Treatment with LYFGENIA may increase your risk of developing blood cancer. Blood cancer can develop many years after treatment with LYFGENIA. Blood cancer can be life-threatening and/or cause death.
Because of the risk of blood cancer, you should talk to your doctor about the benefits and risks of LYFGENIA, and about your treatment options. Your doctor may evaluate if you have risk factors that increase your chances of developing blood cancer after LYFGENIA.
Because of the risk of cancer, it is important for you to be monitored at least every 6 months for a minimum of 15 years after LYFGENIA. Monitoring will include blood tests that measure your blood cell counts and evaluation of the blood cells where the gene product is present with specialized tests. If these tests are abnormal, additional testing may be recommended by your doctor. Additional testing might include more frequent blood tests to watch you more closely for changes in your blood. Additional testing could also include a bone marrow evaluation, which can tell your doctor if a blood cancer is developing.
Blood cancer may cause no symptoms, or symptoms can be general. You or your caregiver should call your healthcare provider right away for any of these signs or symptoms:- Abnormal bruising or bleeding (including nosebleed)
- Blood in urine, stool, or vomit
- Coughing up blood
- Severe headache
- Unusual stomach or back pain
- Fever (100.4°F/38°C or higher)
- Swollen glands
- Abnormal tiredness
You may experience side effects associated with other medicines administered as part of the LYFGENIA treatment regimen. Talk to your physician regarding those possible side effects. Your healthcare providers may give you other medicines to treat your side effects.
It is important that you or your caregiver tell your healthcare providers that you have received LYFGENIA.What is LYFGENIA?
LYFGENIA is a one-time gene therapy to treat sickle cell disease. Sickle cell disease is a genetic, inherited, lifelong disease caused by an alteration in one of the genes in the red blood cell, the beta-globin gene, that causes the normal disc-shaped red cells to take the shape of a sickle, causing anemia and vaso-occlusive events, like a pain crisis. LYFGENIA is made specifically for each patient, using the patient's own blood stem cells (from which red blood cells are produced). It adds functional copies of the beta-globin gene to your cells leading to production of anti-sickling hemoglobin that may decrease or stop vaso-occlusive events.How will I get LYFGENIA?
Before treatment: Your healthcare providers will give you other medicines, including a chemotherapy medicine (given in the vein), as part of your treatment with LYFGENIA. It's important to talk to your healthcare provider about the risks and benefits of all medicines involved in your treatment. You will be admitted to a treatment center during this process (see Step 3).
After receiving the chemotherapy, it may not be possible for you to become pregnant or father a child. You should consider discussing options for fertility preservation with your doctor before treatment.
STEP 1: LYFGENIA is made specifically for you from your own blood stem cells. Your healthcare provider will collect your blood stem cells through a procedure/process called mobilization and apheresis (A-feh-REE-sis). This process takes approximately one week and may need to be repeated to obtain a sufficient number of cells.
'Back-up' stem cells (or 'rescue cells') are also collected and stored at the treatment center. This is a precaution in case there is a problem in the treatment process. If this happens, your back-up stem cells will be given back to you. If you receive back-up cells, you will have no benefit from LYFGENIA.
STEP 2: Your blood stem cells will be sent to a manufacturing site where they are used to make your LYFGENIA. It typically takes 10 to 15 weeks from the time your cells are collected to make and test LYFGENIA before it is shipped to your healthcare provider, but the time may vary.
STEP 3: Before you receive LYFGENIA, your healthcare provider will give you chemotherapy for a few days to make room in the bone marrow. You will be admitted to the treatment center for this step and remain there until after LYFGENIA infusion.
STEP 4: LYFGENIA is given by an intravenous infusion (into your vein). You may receive more than one bag of LYFGENIA. Each bag is infused in 30 minutes or less.
After LYFGENIA infusion, you will stay in the treatment center for approximately 3-6 weeks so that your healthcare team can closely monitor your recovery. Your healthcare provider will determine when you can go home.What should I avoid after receiving LYFGENIA? - Do not donate blood, organs, tissues or cells.
What are the possible side effects of LYFGENIA?
The possible side effects of LYFGENIA include:- On the day of treatment with LYFGENIA
- Low blood pressure
- Hot flush
- Following treatment
- Blood cancer. Refer to "What is the most important information I should know about LYFGENIA?"
- Longer time for platelets to recover, which may reduce the ability of blood to clot and may cause bleeding
General information about the safe and effective use of LYFGENIA.
It is important that you have regular check-ups with your healthcare provider, including blood tests at least every 6 months as advised by your health-care provider, to detect any adverse effects and to confirm that LYFGENIA is still working.
Patients treated with LYFGENIA are encouraged to enroll in a post-marketing study to assess the long-term safety of LYFGENIA and the risk of blood cancers occurring after treatment with LYFGENIA. Patients should discuss the option to participate with their physician.
LYFGENIA will not give you a human immunodeficiency virus (HIV) infection. Treatment with LYFGENIA may cause a false-positive HIV test result by some commercial tests (specifically, a PCR-based test). If you need to have an HIV test, talk with your healthcare provider about the appropriate test to use.
Talk to your healthcare provider about any concerns. You can ask your healthcare provider for information about LYFGENIA that is written for healthcare professionals.
For more information, go to LYFGENIA.com or call 1-833-666-2583 for bluebird Patient Services (my bluebird support).
Manufactured for: bluebird bio, Inc., Somerville, Massachusetts 02145 -
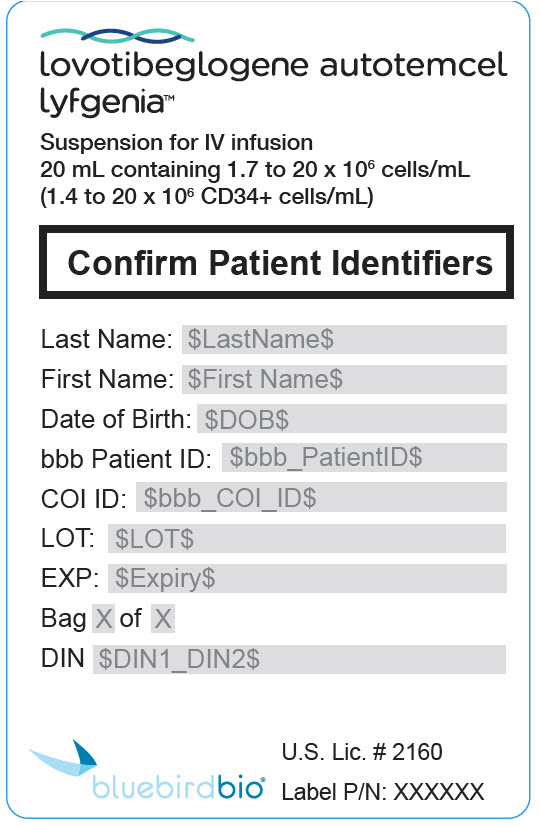
PRINCIPAL DISPLAY PANEL - 20 mL Bag Patient Identifier Label
lovotibeglogene autotemcel
lyfgenia™Suspension for IV infusion
20 mL containing 1.7 to 20 x 106 cells/mL
(1.4 to 20 x 106 CD34+ cells/mL)Confirm Patient Identifiers
Last Name: $LastName$
First Name: $First Name$
Date of Birth: $DOB$
bbb Patient ID: $bbb_PatientID$
COI ID: $bbb_COI_ID$
LOT: $LOT$
EXP: $Expiry$
Bag X of X
DIN $DIN1_DIN2$
U.S. Lic. # 2160
Label P/N: XXXXXX
bluebirdbio®
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Label
lovotibeglogene autotemcel
lyfgenia™Suspension for IV infusion
20 mL containing 1.7 to 20 x 106 cells/mL
(1.4 to 20 x 106 CD34+ cells/mL)For autologous use only. For intravenous use only. Rx only.
Contains genetically modified autologous hematopoietic stem cells
suspended in cryopreservation solution containing 5% DMSO.Not evaluated for infectious substances.
Do not irradiate. Do not use an in-line blood filter or infusion pump.
See full prescribing information for dosage and administration.
See Lot Information Sheet for number of infusion bags and CD34+
cells per kg for this patient. Dispense with Medication Guide.P/N: XXXXXXX
Label P/N: XXXXXXXManufactured for: bluebird bio, Inc.
Somerville, MA 02145
-
PRINCIPAL DISPLAY PANEL - 20 mL Bag Cassette Label
lovotibeglogene autotemcel
lyfgenia™Suspension for IV infusion
20 mL containing 1.7 to 20 x 106 cells/mL
(1.4 to 20 x 106 CD34+ cells/mL)For autologous use only. For intravenous use only. Rx only.
Contains genetically modified autologous hematopoietic stem cells
suspended in cryopreservation solution containing 5% DMSO.Keep infusion bag(s) in the metal cassette(s). Store in the vapor phase of
liquid nitrogen at ≤ -140°C until ready for thaw and administration. Once
thawed do not re-freeze.See full prescribing information for dosage and administration.
Do not irradiate. Do not use an in-line blood filter or infusion pump.
Not evaluated for infectious substances. No preservatives.
See Lot Information Sheet for number of infusion bags and CD34+ cells
per kg for this patient. Dispense with Medication Guide.Confirm Patient Identifiers
Last Name: $LastName$
First Name: $FirstName$
Date of Birth: $DOB$
bbb Patient ID: $bbb_PatientID$
COI ID: $bbb_COI_ID$
DIN $DIN1_DIN2$
LOT: $LOT$
EXP: $Expiry$
Bag Xof X
U.S.Lic. # 2160
Manufactured for: bluebird bio, Inc.
Somerville, MA 02145
1-833-999-6378
LYFGENIA.comP/N: XXXXXXX
Label P/N: XXXXXXbluebirdbio®

-
INGREDIENTS AND APPEARANCE
LYFGENIA
lovotibeglogene autotemcel suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC:73554-1111 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lovotibeglogene autotemcel (UNII: 2C6A9NH2Z8) (lovotibeglogene autotemcel - UNII:2C6A9NH2Z8) lovotibeglogene autotemcel 20000000 in 20 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73554-1111-1 1 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125788 12/08/2023 Labeler - bluebird bio, Inc. (969116102) Establishment Name Address ID/FEI Business Operations Minaris Regenerative Medicine, LLC 117721509 ANALYSIS(73554-1111) , LABEL(73554-1111) , MANUFACTURE(73554-1111) , PACK(73554-1111) , API MANUFACTURE(73554-1111)