Label: NEUTROGENA SKINCLEARING MINERAL POWDER HONEY 85- salicylic acid powder
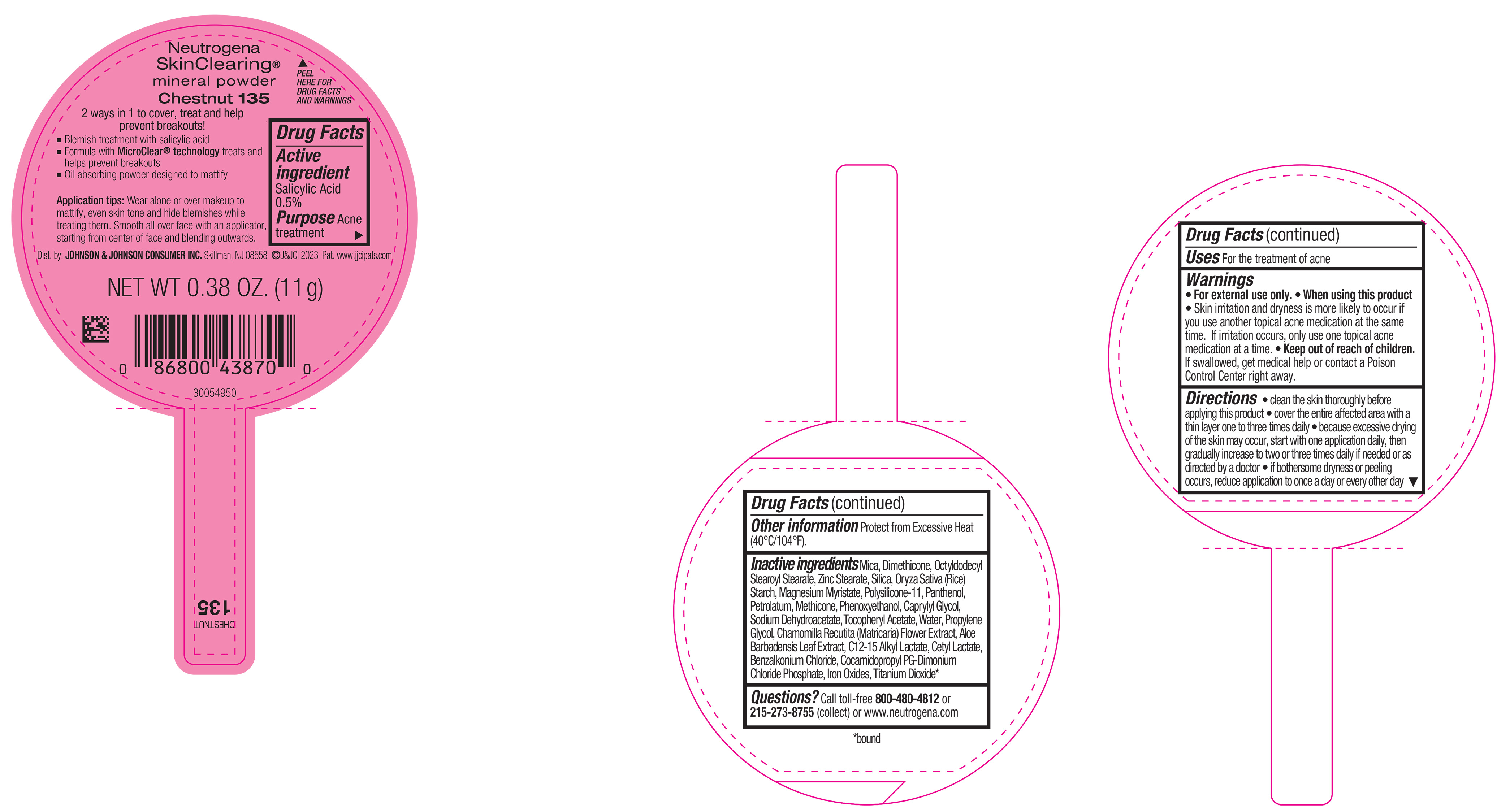
NEUTROGENA SKINCLEARING MINERAL POWDER CHESTNUT 135- salicylic acid powder
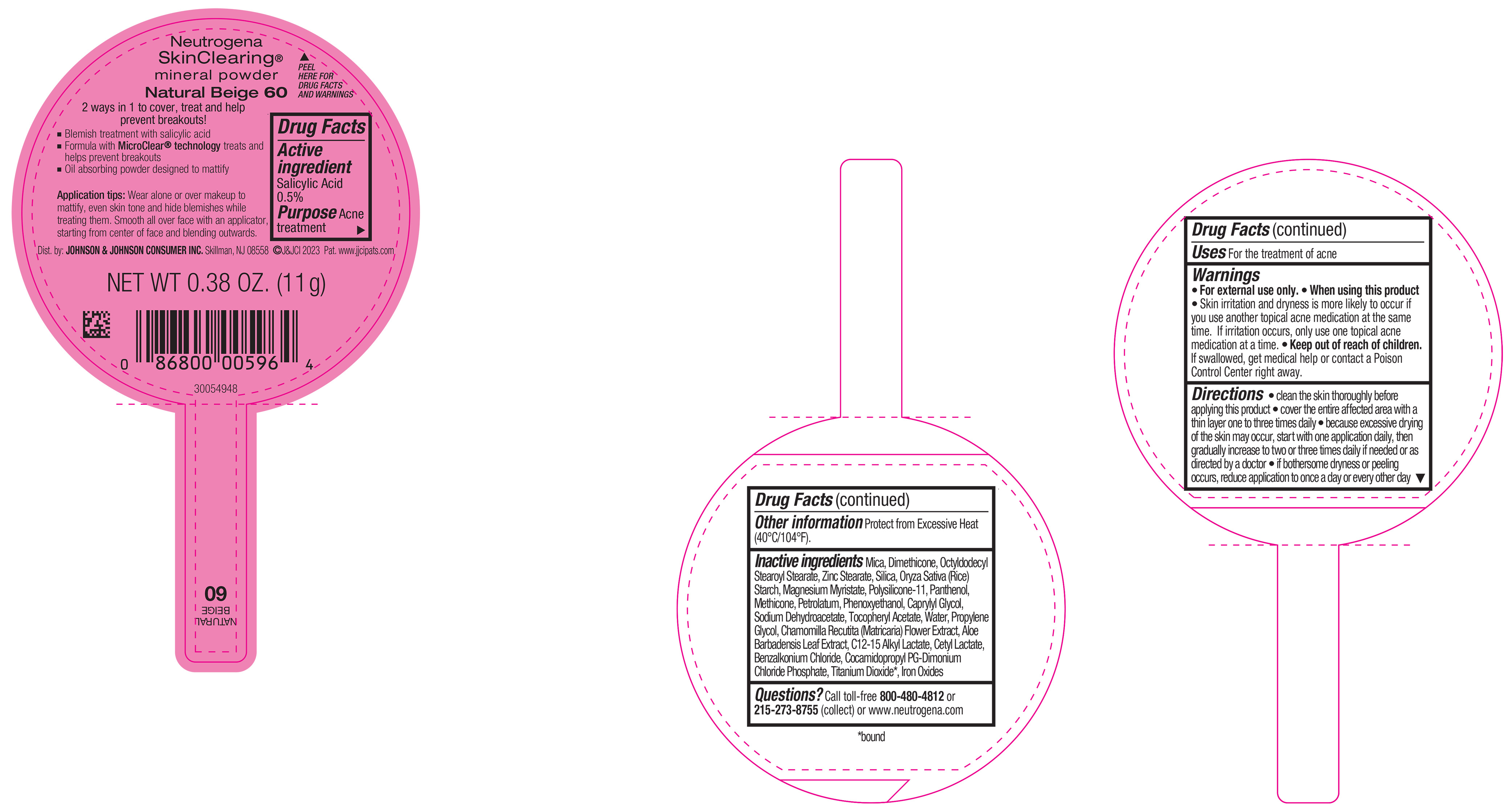
NEUTROGENA SKINCLEARING MINERAL POWDER NATURAL BEIGE 60- salicylic acid powder
NEUTROGENA SKINCLEARING MINERAL POWDER SOFT BEIGE 50- salicylic acid powder
NEUTROGENA SKINCLEARING MINERAL POWDER NATURAL IVORY 20- salicylic acid powder
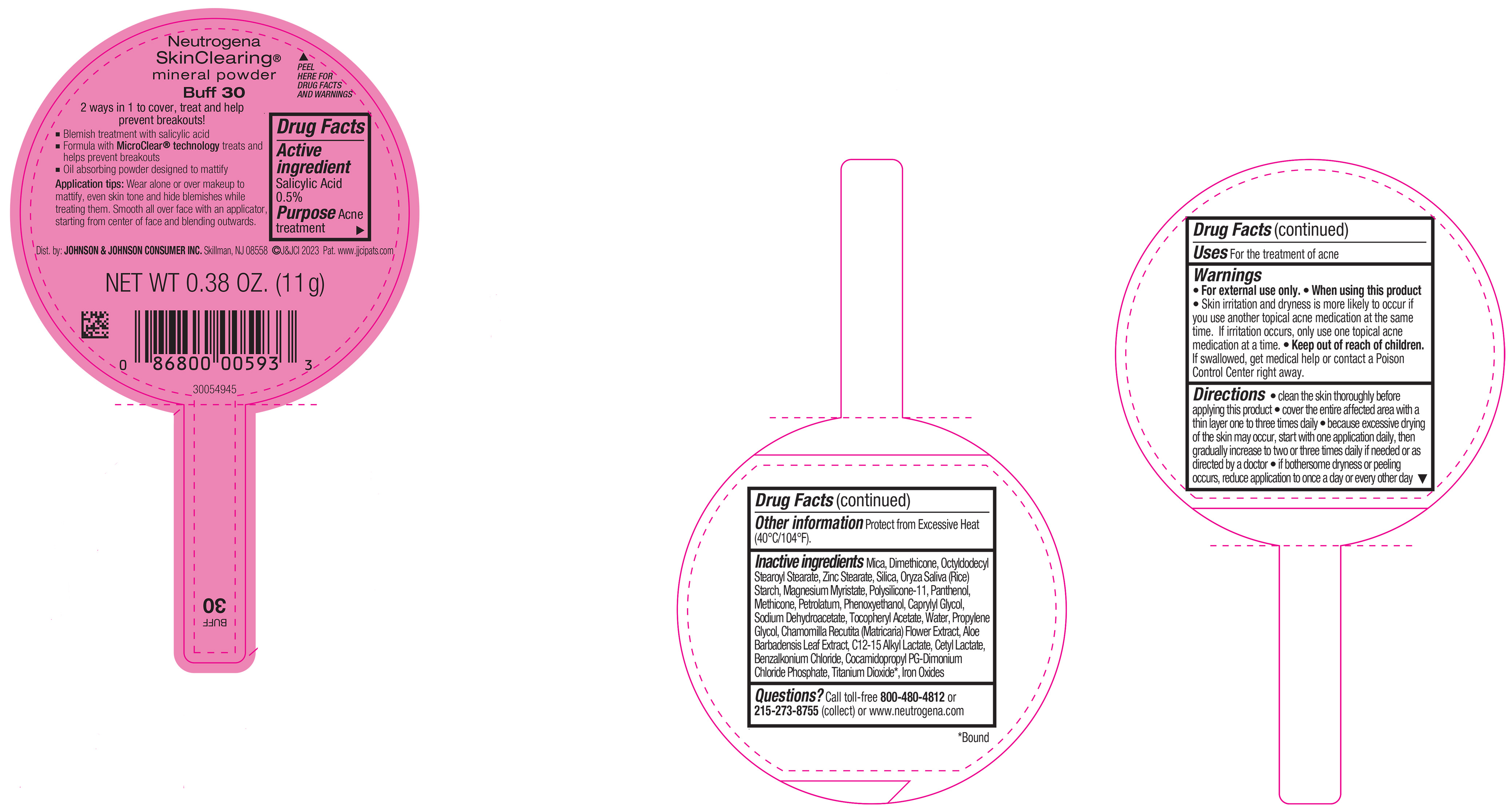
NEUTROGENA SKINCLEARING MINERAL POWDER BUFF 30- salicylic acid powder
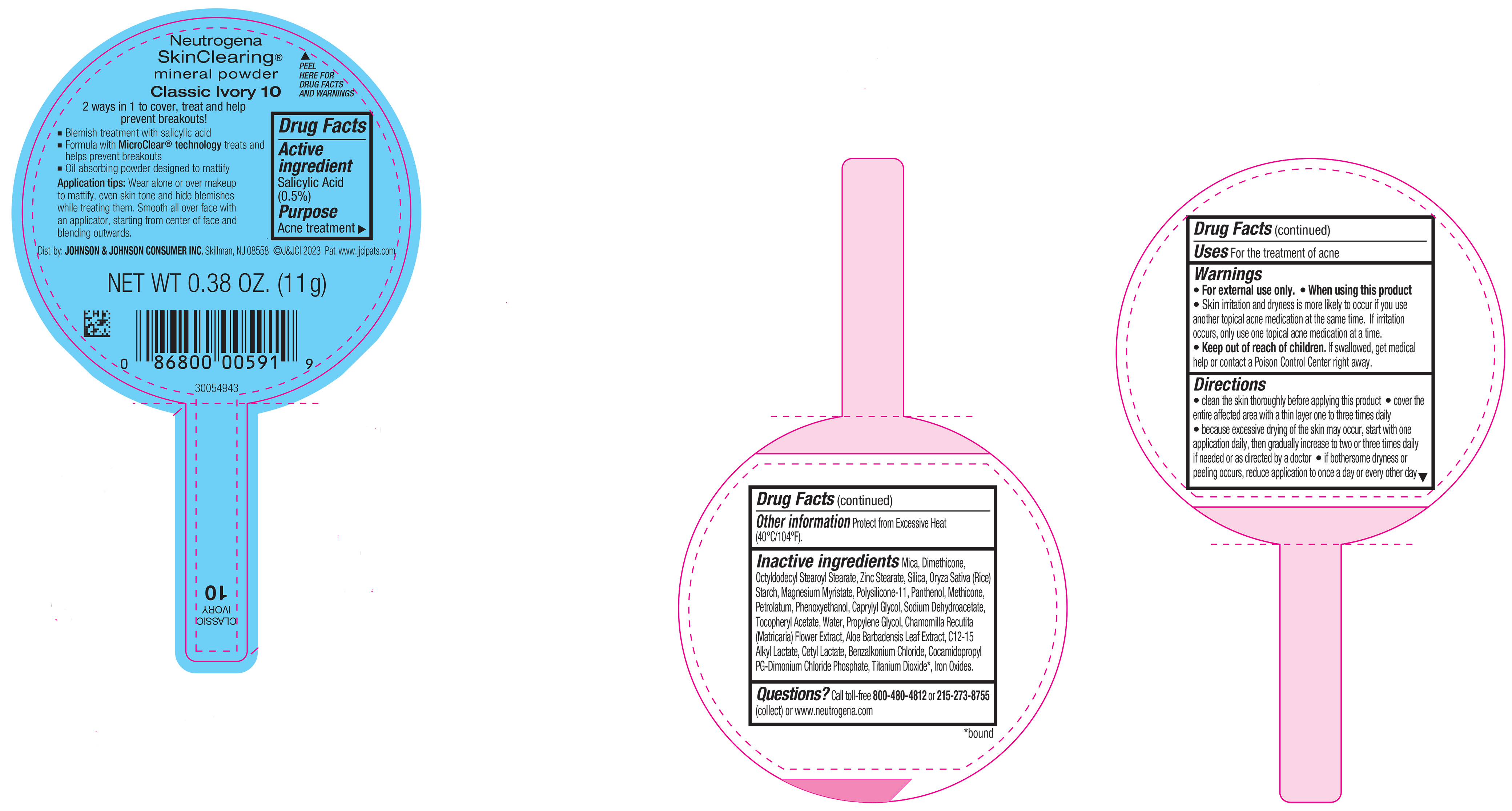
NEUTROGENA SKINCLEARING MINERAL POWDER CLASSIC IVORY 10- salicylic acid powder
NEUTROGENA SKINCLEARING MINERAL POWDER NUDE 40- salicylic acid powder
-
NDC Code(s):
69968-0833-1,
69968-0834-1,
69968-0835-1,
69968-0836-1, view more69968-0837-1, 69968-0838-1, 69968-0839-1, 69968-0840-1
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
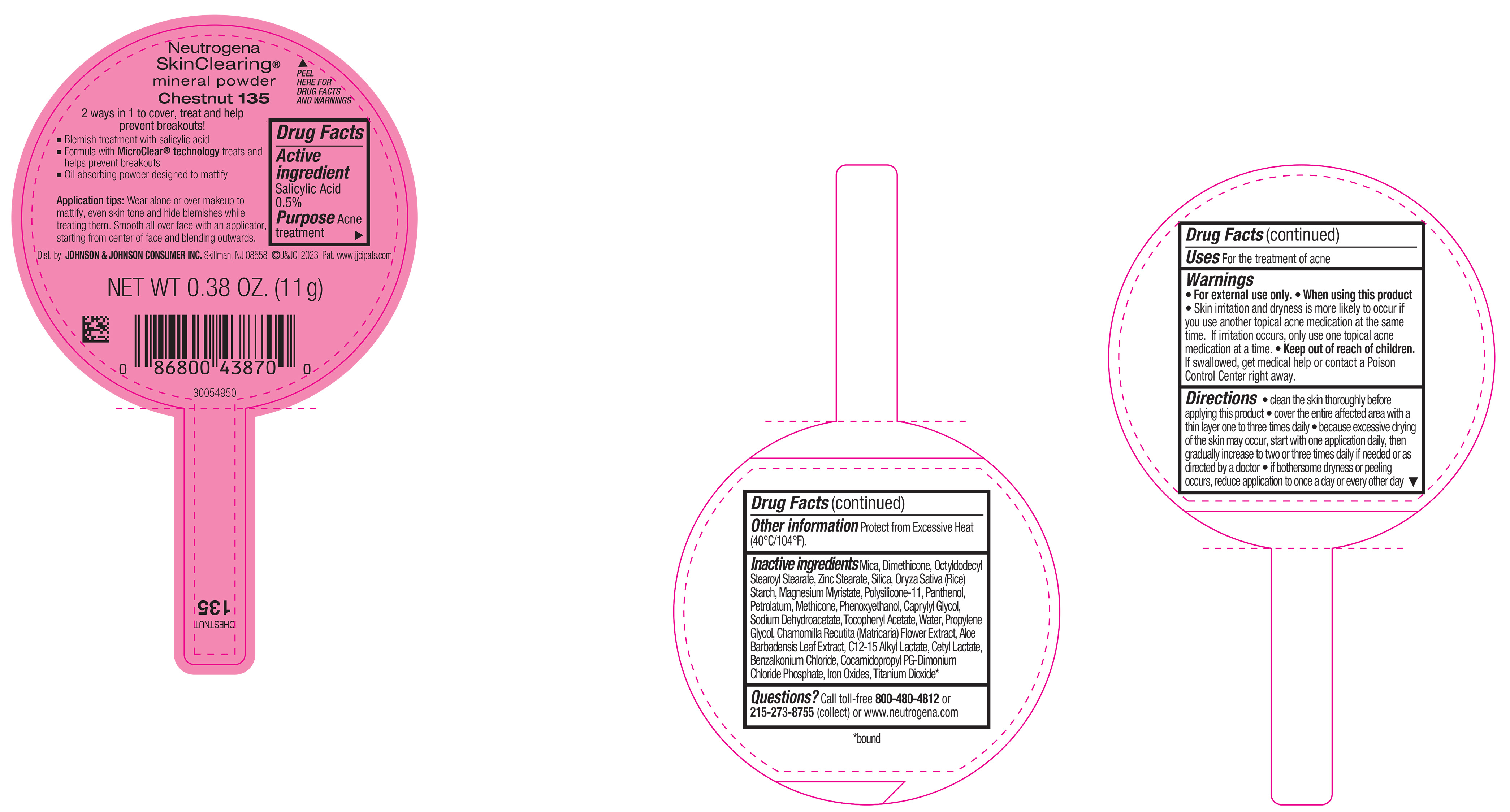
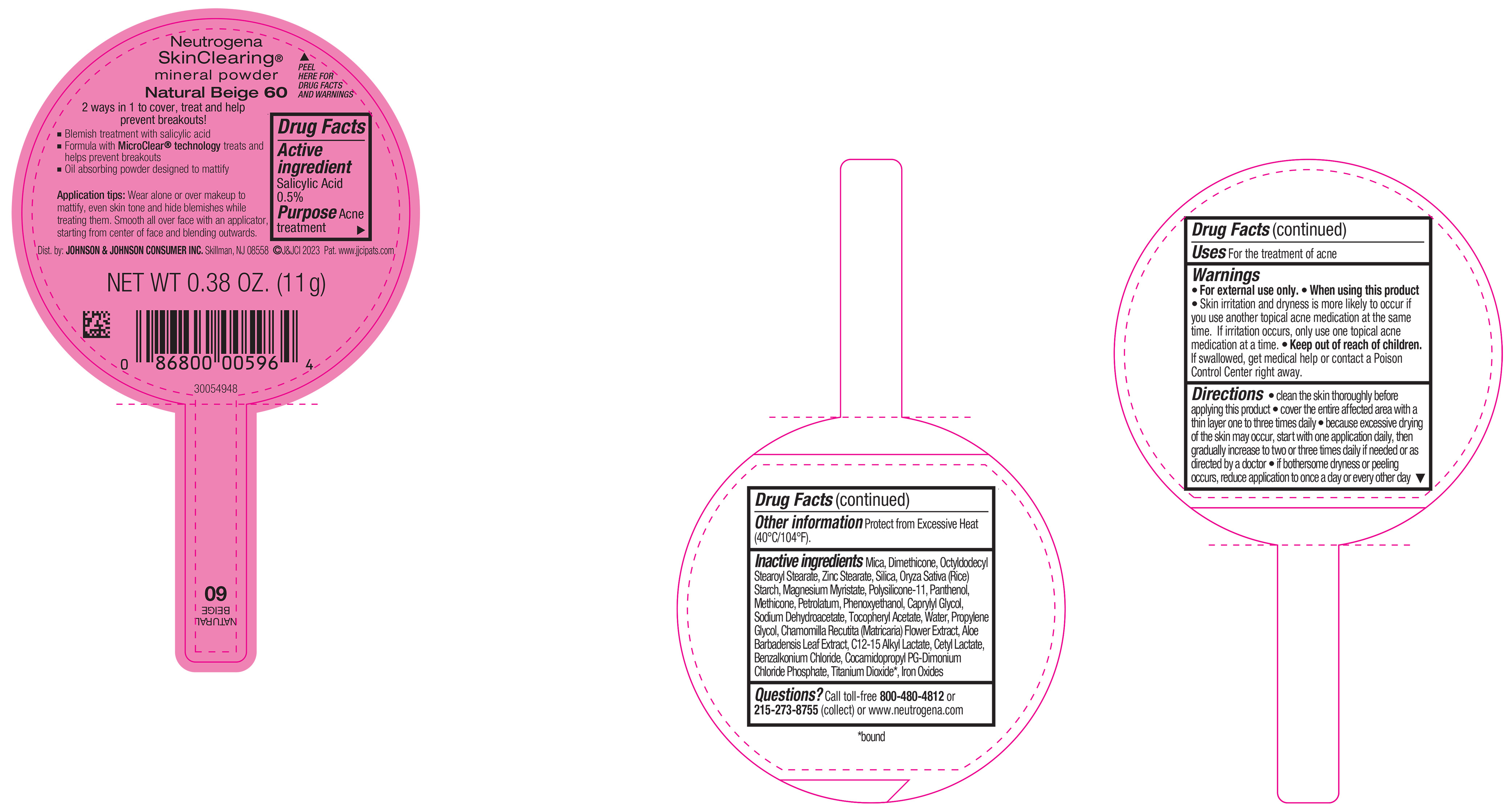
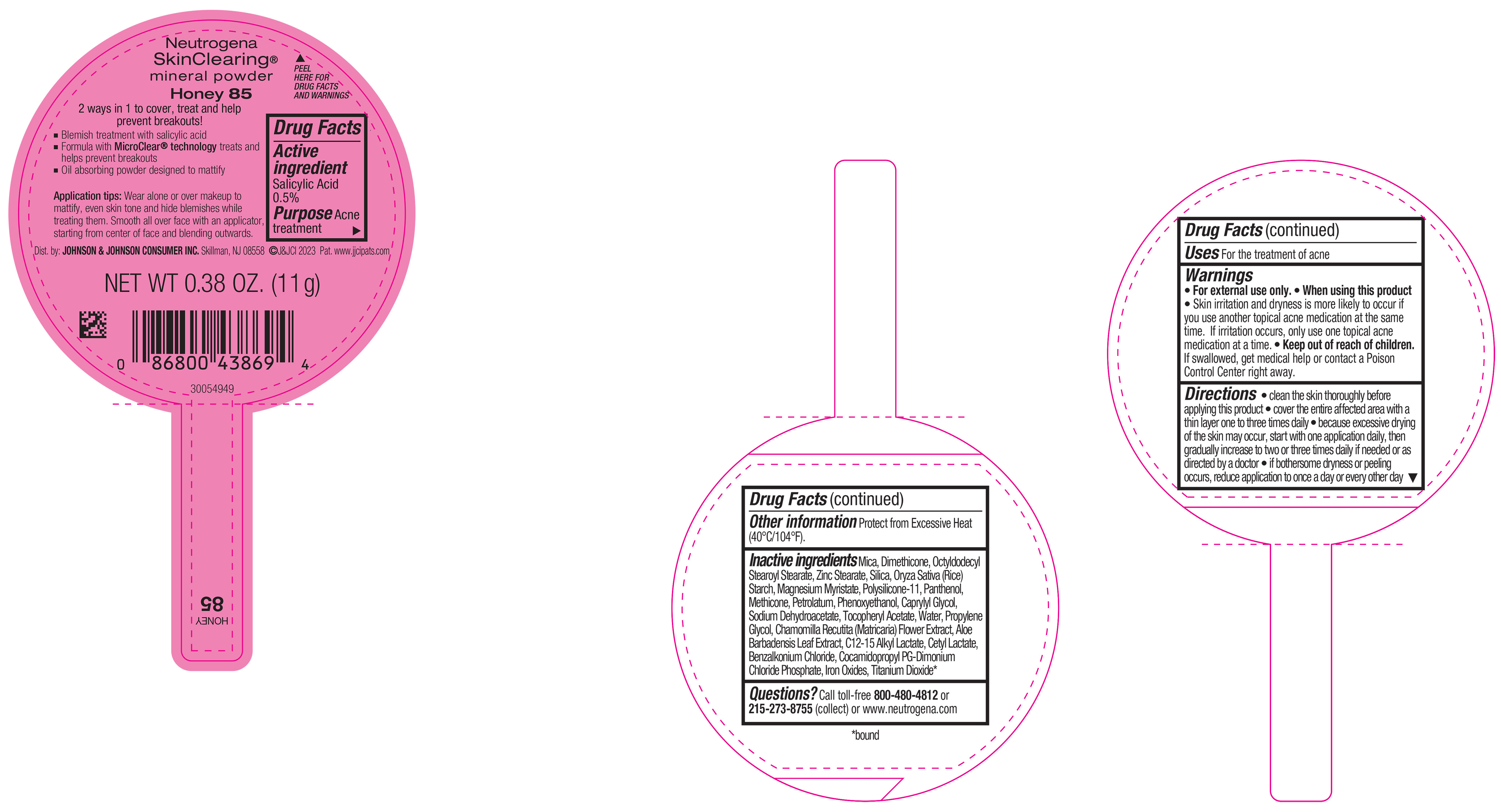
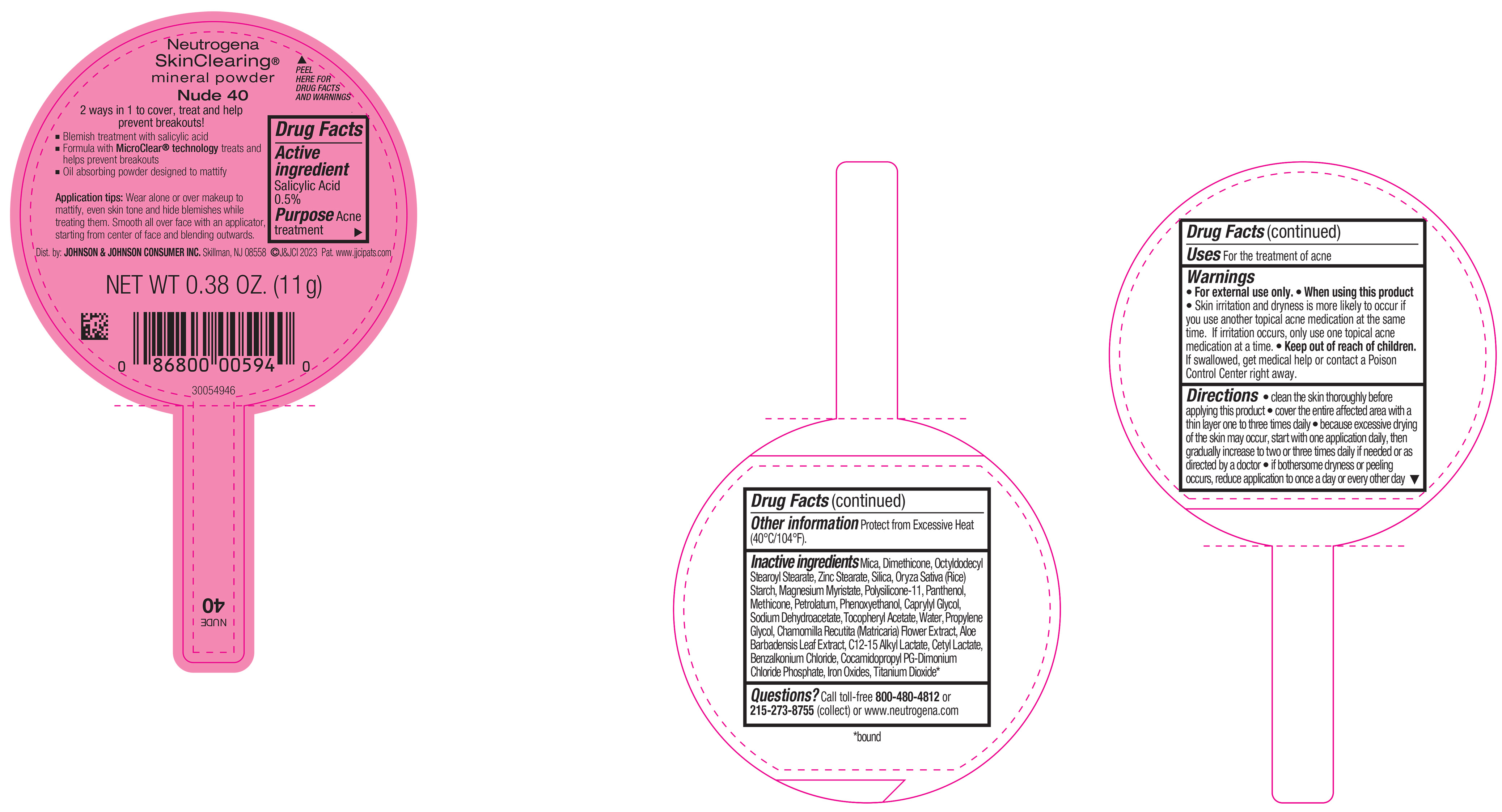
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
• clean the skin thoroughly before applying this product
• cover the entire affected area with a thin layer one to three times daily
• because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
• if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other Information
-
Inactive ingredients
Mica, Dimethicone, Octyldodecyl Stearoyl Stearate, Zinc Stearate, Silica, Oryza Sativa (Rice) Starch, Magnesium Myristate, Polysilicone-11, Panthenol, Petrolatum, Methicone, Phenoxyethanol, Caprylyl Glycol, Sodium Dehydroacetate, Tocopheryl Acetate, Water, Propylene Glycol, Chamomilla Recutita (Matricaria) Flower Extract, Aloe Barbadensis Leaf Extract, C12-15 Alkyl Lactate, Cetyl Lactate, Benzalkonium Chloride, Cocamidopropyl PG-Dimonium Chloride Phosphate, Iron Oxides, Titanium Dioxide*.
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Chestnut 135
Neutrogena
SkinClearing ®
mineral powder
Chestnut 135
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Natural Beige 60
Neutrogena
SkinClearing ®
mineral powder
Natural Beige 60
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Honey 85
Neutrogena
SkinClearing ®
mineral powder
Honey 85
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
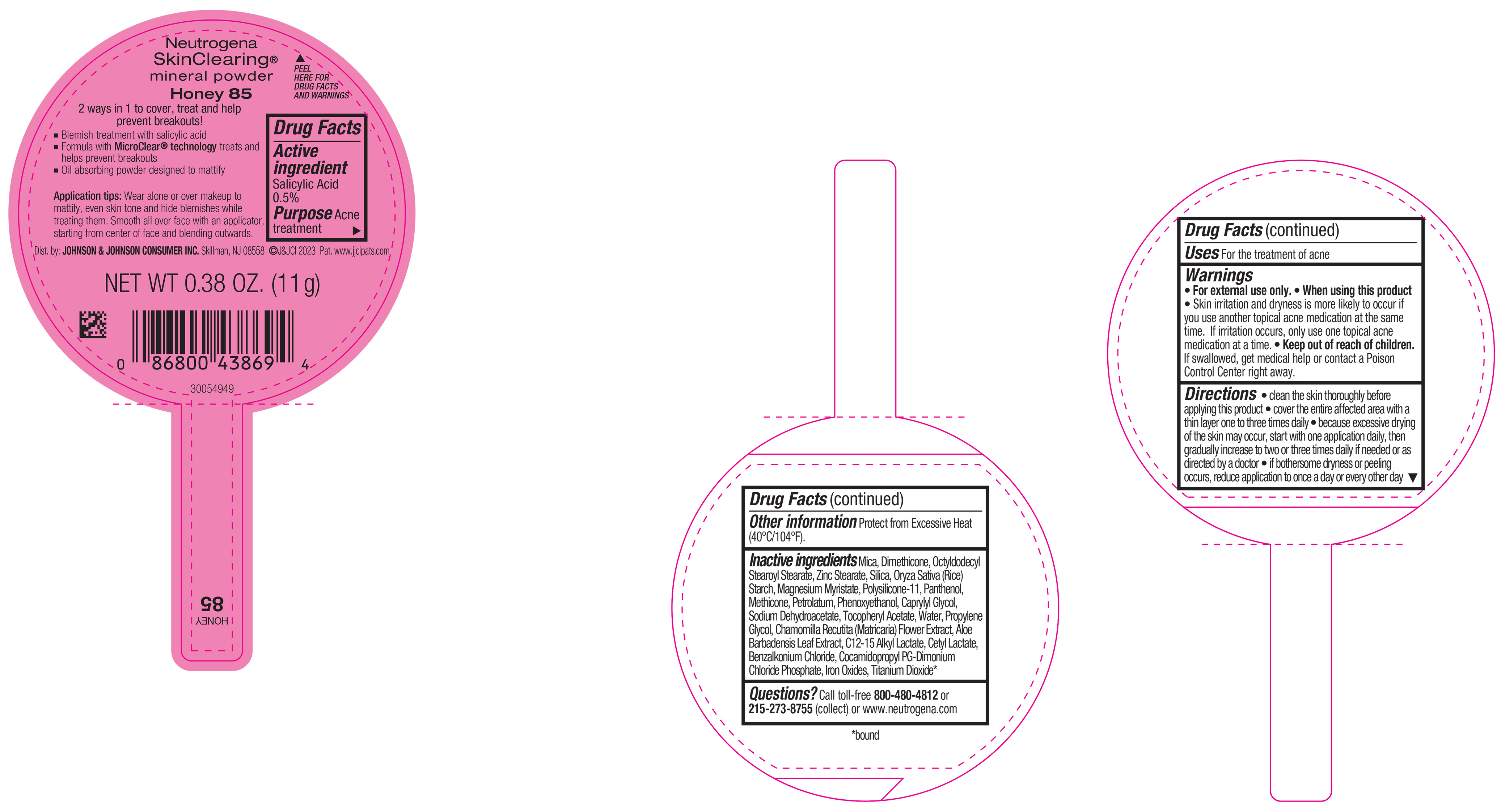
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Soft Beige 50
Neutrogena
SkinClearing ®
mineral powder
Soft Beige 50
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
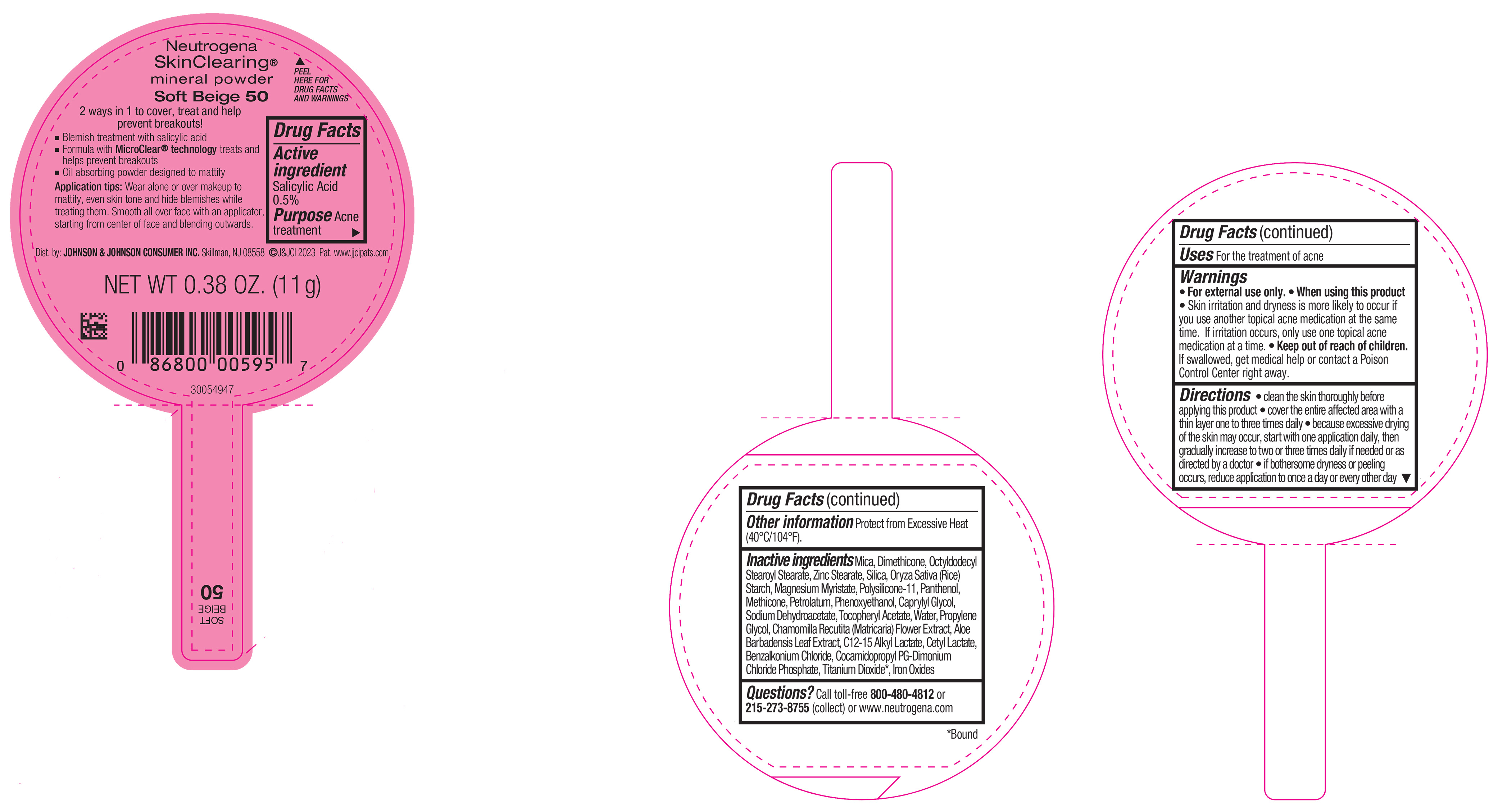
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Nude 40
Neutrogena
SkinClearing ®
mineral powder
Nude 40
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
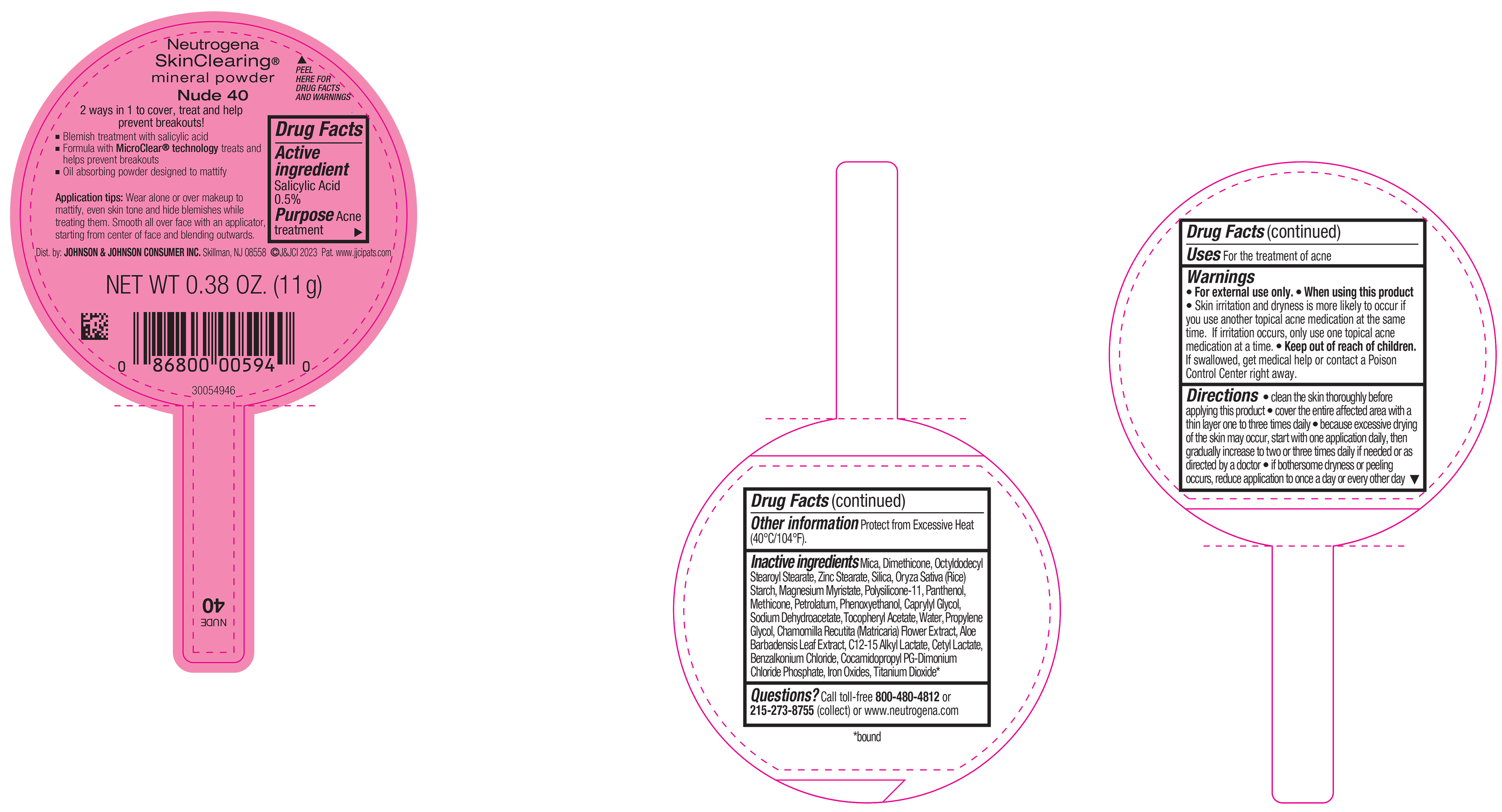
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Buff 30
Neutrogena
SkinClearing ®
mineral powder
Buff 30
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
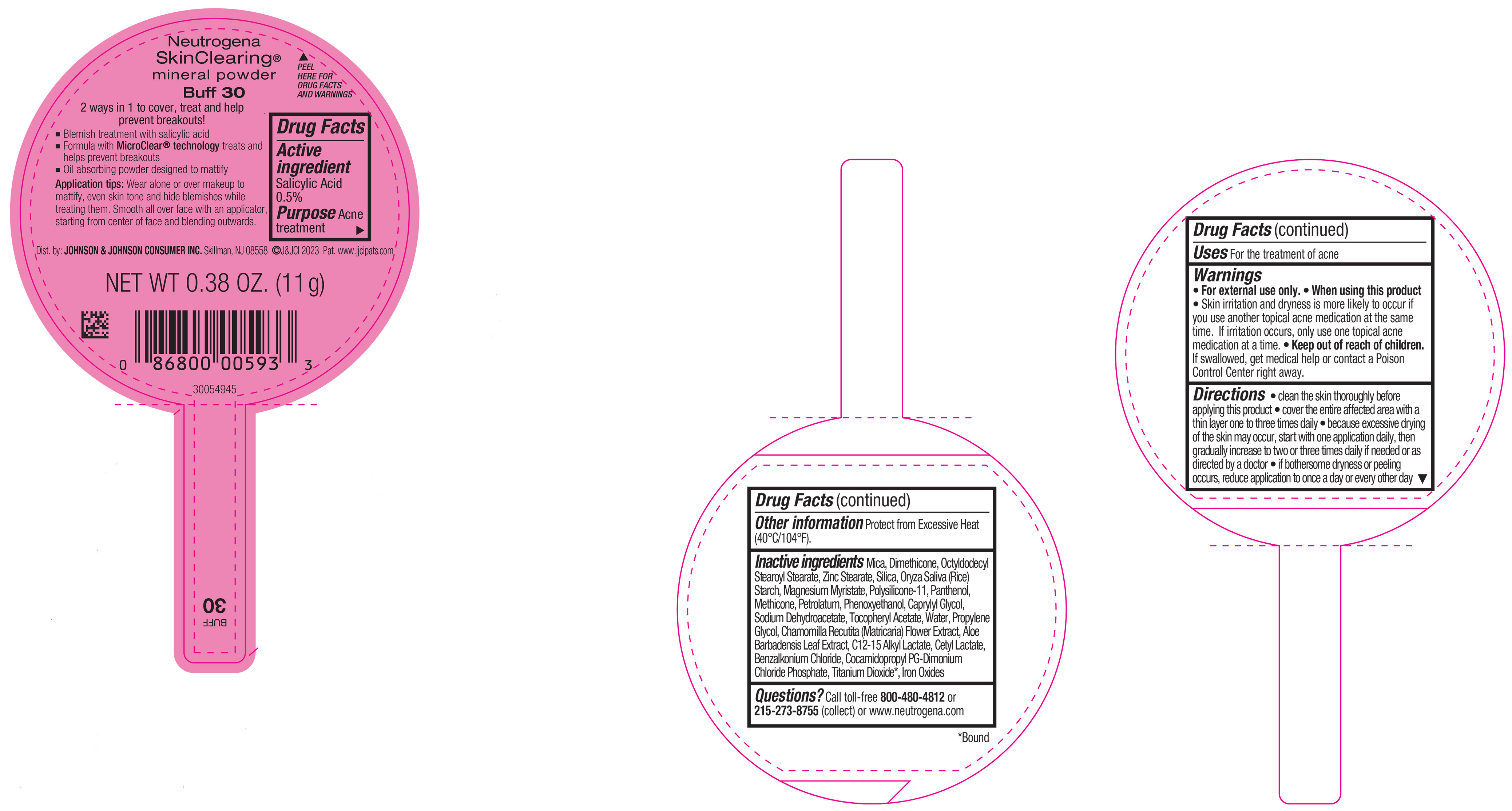
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Natural Ivory 20
Neutrogena
SkinClearing ®
mineral powder
Natural Ivory 20
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
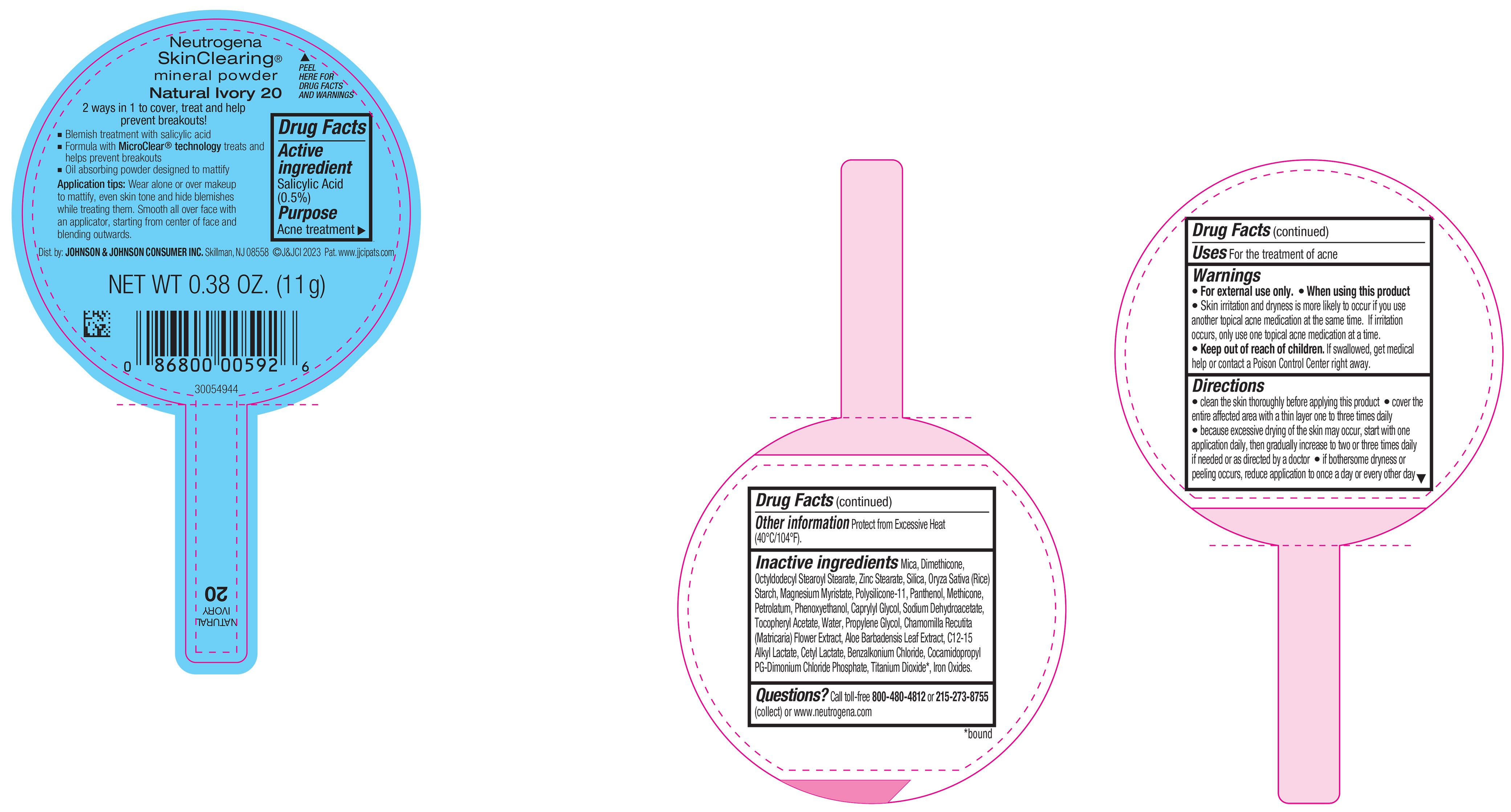
-
PRINCIPAL DISPLAY PANEL - 11 g Container Label - Classic Ivory 10
Neutrogena
SkinClearing ®
mineral powder
Classic Ivory 10
2 ways in 1 to cover, treat and help
prevent breakouts!
▪ Blemish treatment with salicylic acid
▪ Formula with MicroClear ® technology treats and
helps prevent breakouts
▪ Oil absorbing powder designed to mattify
Application tips: Wear alone or over makeup to
mattify, even skin tone and hide blemishes while
treating them. Smooth all over face with an applicator,
starting from center of face and blending outwards.
NET WT 0.38 OZ. (11 g)
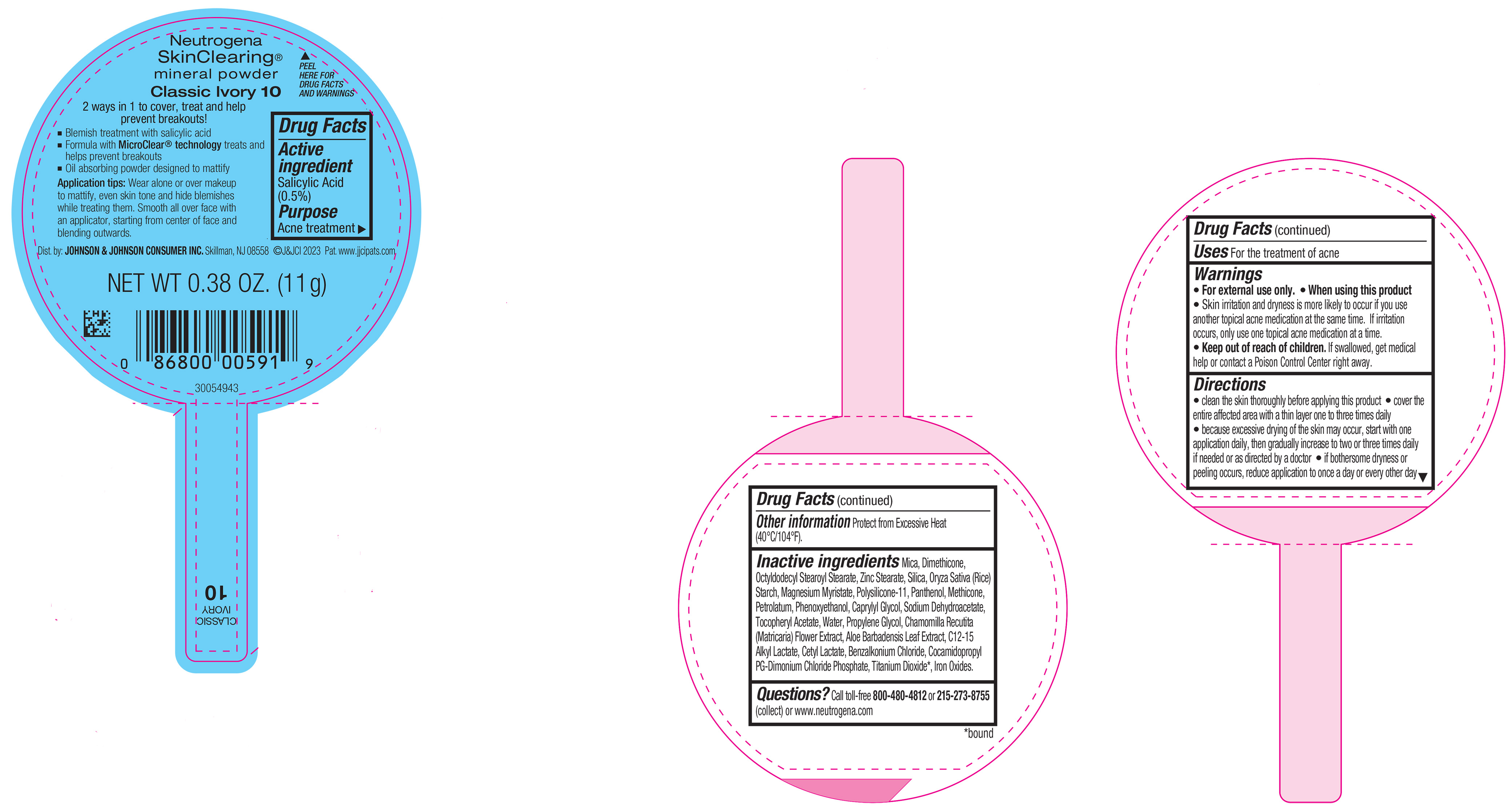
-
INGREDIENTS AND APPEARANCE
NEUTROGENA SKINCLEARING MINERAL POWDER HONEY 85
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0834 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0834-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER CHESTNUT 135
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0833 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0833-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER NATURAL BEIGE 60
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0835 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0835-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER SOFT BEIGE 50
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0836 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0836-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER NATURAL IVORY 20
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0839 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0839-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER BUFF 30
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0838 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0838-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER CLASSIC IVORY 10
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0840 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0840-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 NEUTROGENA SKINCLEARING MINERAL POWDER NUDE 40
salicylic acid powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0837 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMOMILE (UNII: FGL3685T2X) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHICONE (20 CST) (UNII: 6777U11MKT) PETROLATUM (UNII: 4T6H12BN9U) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) STARCH, RICE (UNII: 4DGK8B7I3S) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PANTHENOL (UNII: WV9CM0O67Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CETYL LACTATE (UNII: A7EVH2RK4O) FERRIC OXIDE RED (UNII: 1K09F3G675) OCTYLDODECYL STEAROYL STEARATE (UNII: 3D47Q6D93C) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0837-1 11 g in 1 CONTAINER; Type 0: Not a Combination Product 01/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/25/2024 Labeler - Kenvue Brands LLC (118772437)